Label: PETROLEUM- petrolatum jelly
-
NDC Code(s):
61734-040-03,
61734-040-04,
61734-040-05,
61734-040-06, view more61734-040-07, 61734-040-08, 61734-040-09
- Packager: Delon Laboratories (1990) Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
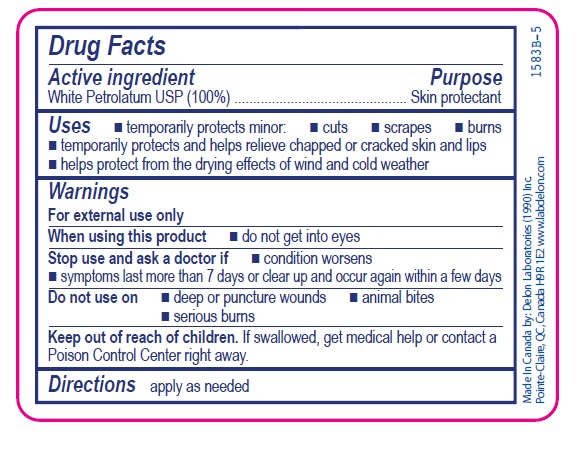
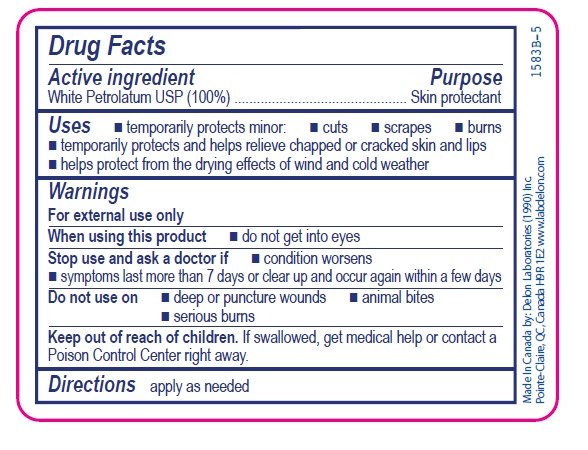
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- INACTIVE INGREDIENT
- Delon Petroleum Jelly USP 368g
-
INGREDIENTS AND APPEARANCE
PETROLEUM
petrolatum jellyProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61734-040 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 100 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61734-040-03 368 g in 1 JAR; Type 0: Not a Combination Product 05/07/2010 11/30/2025 2 NDC:61734-040-04 212 g in 1 JAR; Type 0: Not a Combination Product 05/07/2010 08/31/2021 3 NDC:61734-040-05 113 g in 1 JAR; Type 0: Not a Combination Product 10/04/2014 12/04/2014 4 NDC:61734-040-06 10 g in 1 TUBE; Type 0: Not a Combination Product 10/04/2014 12/04/2014 5 NDC:61734-040-07 100 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/07/2010 11/08/2017 6 NDC:61734-040-08 105 g in 1 JAR; Type 0: Not a Combination Product 10/04/2014 12/04/2014 7 NDC:61734-040-09 255 g in 1 JAR; Type 0: Not a Combination Product 10/04/2014 12/04/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 05/07/2010 11/30/2025 Labeler - Delon Laboratories (1990) Ltd (248364184) Establishment Name Address ID/FEI Business Operations Laboratoires Delon 208896216 pack(61734-040) , manufacture(61734-040) , label(61734-040)