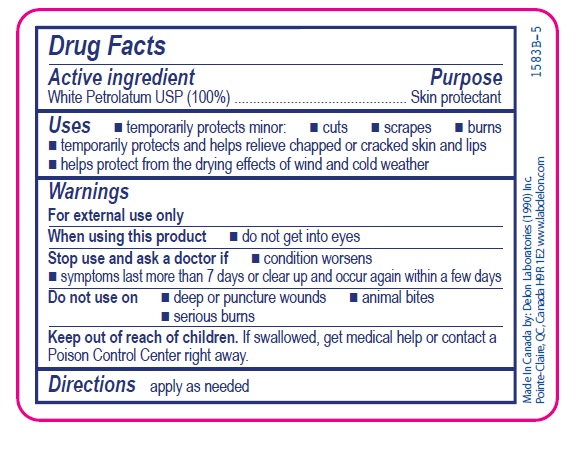
Active ingredient
White Petrolatum USP (100%)
Uses
- temporarily protects minor:
- cuts
- scrapes
- burns
- temporarily protects and helps relieve chapped or cracked skin and lips
- helps protect from the drying effects of wind and cold weather
Warnings
For external use only
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Do not use on
- deep or puncture wounds
- animal bites
- serious burns
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
apply as needed
Delon Petroleum Jelly USP 368g