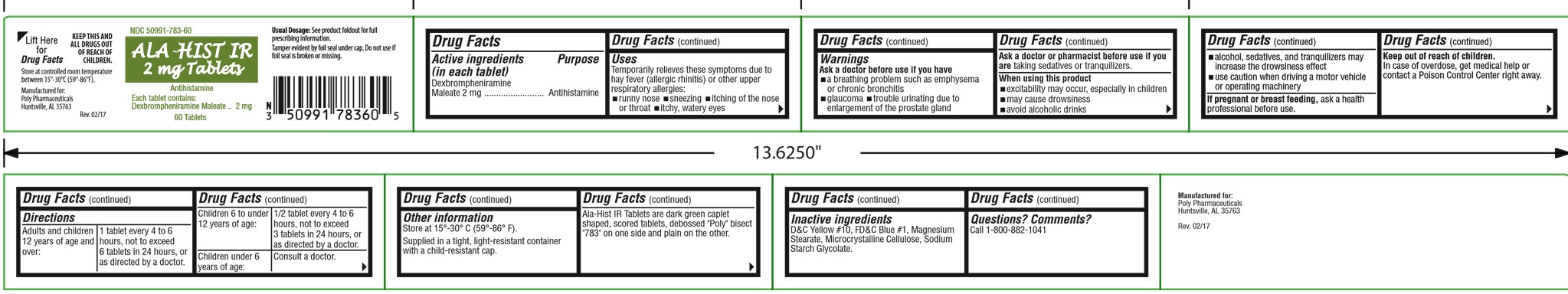
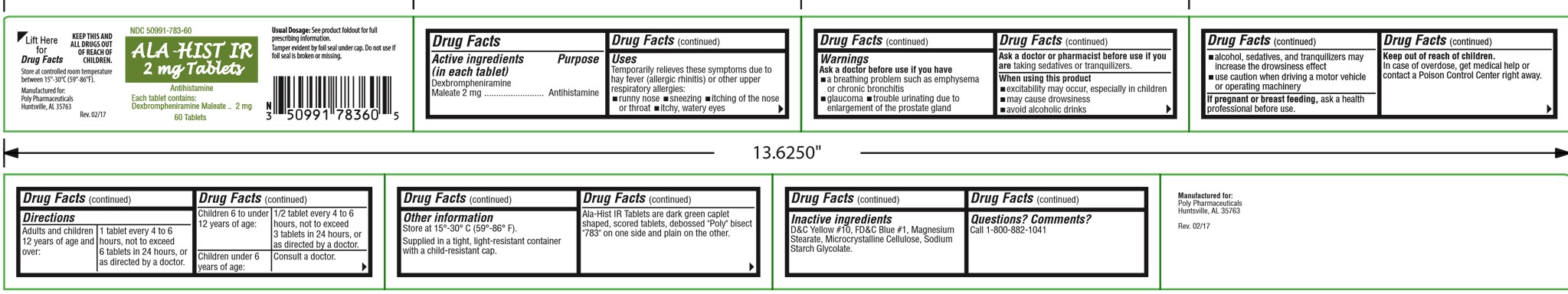
Label: ALA-HIST IR- dexbrompheniramine maleate tablet
- NDC Code(s): 50991-783-02, 50991-783-60
- Packager: Poly Pharmaceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Uses
-
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers.
- Directions
-
Other information
Store at 15° - 30°C (59° - 86°F).
Supplied in a tight, light-resistant container with a child-resistant cap.
Contains color additives including FD&C Yellow No. 5 (tartrazine).
Ala-Hist IR Tablets are dark green caplet shaped, scored tablets, debossed "Poly" bisect "783" on one side and plain on the other.
- Inactive ingredients
- Questions?
-
Product Packaging
The packaging below represents the labeling currently used.
Principal display panel and side panel for 60 tablets label:
NDC 50991-783-60
ALA-HIST IR
2 mg TabletsAntihistamine
Each tablet contains:
Dexbrompheniramine Maleate.........2 mg60 Tablets
Usual Dosage: See product foldout for full
prescribing informationTamper evident by foil seal under cap. Do not
use if foil seal is broken or missing.
KEEP THIS AND ALL DRUGS OUT OF
REACH OF CHILDREN.
Store at controlled room temperature 15°-30°C
(59°-86°F).Manufactured for:
Poly PharmaceuticalsRev. 02/12

-
INGREDIENTS AND APPEARANCE
ALA-HIST IR
dexbrompheniramine maleate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50991-783 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXBROMPHENIRAMINE MALEATE (UNII: BPA9UT29BS) (DEXBROMPHENIRAMINE - UNII:75T64B71RP) DEXBROMPHENIRAMINE MALEATE 2 mg Inactive Ingredients Ingredient Name Strength CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color green (dark green) Score 2 pieces Shape OVAL Size 11mm Flavor Imprint Code Poly;783 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50991-783-02 12 in 1 CARTON 08/22/2011 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:50991-783-60 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/22/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/22/2011 Labeler - Poly Pharmaceuticals, Inc. (198449894)