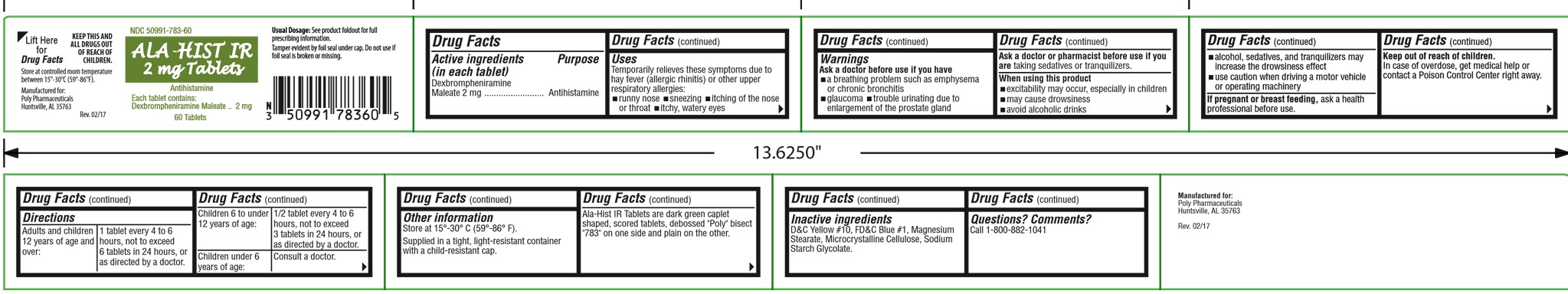
Uses
Temporarily relieves these symptoms due to hay fever (allergic rhinitis) or upper respiratory allergies:
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers.
Directions
| Adults and children
12 years of age and over: | 1 tablet every 4 to 6
hours, not to exceed 6 tablets in 24 hours. |
| Children 6 to under
12 years of age: | 1/2 tablet every 4 to 6
hours, not to exceed 3 tablets in 24 hours. |
| Children under 6
years of age: | Consult a doctor. |
Other information
Store at 15° - 30°C (59° - 86°F).
Supplied in a tight, light-resistant container with a child-resistant cap.
Contains color additives including FD&C Yellow No. 5 (tartrazine).
Ala-Hist IR Tablets are dark green caplet shaped, scored tablets, debossed "Poly" bisect "783" on one side and plain on the other.
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, dibasic calcium phosphate dihydrate, FD&C Blue #1 aluminum lake, FD&C Yellow #5 aluminum lake, magnesium stearate, and silicified microcrystalline cellulose.
Product Packaging
The packaging below represents the labeling currently used.
Principal display panel and side panel for 60 tablets label:
NDC 50991-783-60
ALA-HIST IR
2 mg Tablets
Antihistamine
Each tablet contains:
Dexbrompheniramine Maleate.........2 mg
60 Tablets
Usual Dosage: See product foldout for full
prescribing information
Tamper evident by foil seal under cap. Do not
use if foil seal is broken or missing.
KEEP THIS AND ALL DRUGS OUT OF
REACH OF CHILDREN.
Store at controlled room temperature 15°-30°C
(59°-86°F).
Manufactured for:
Poly Pharmaceuticals
Rev. 02/12