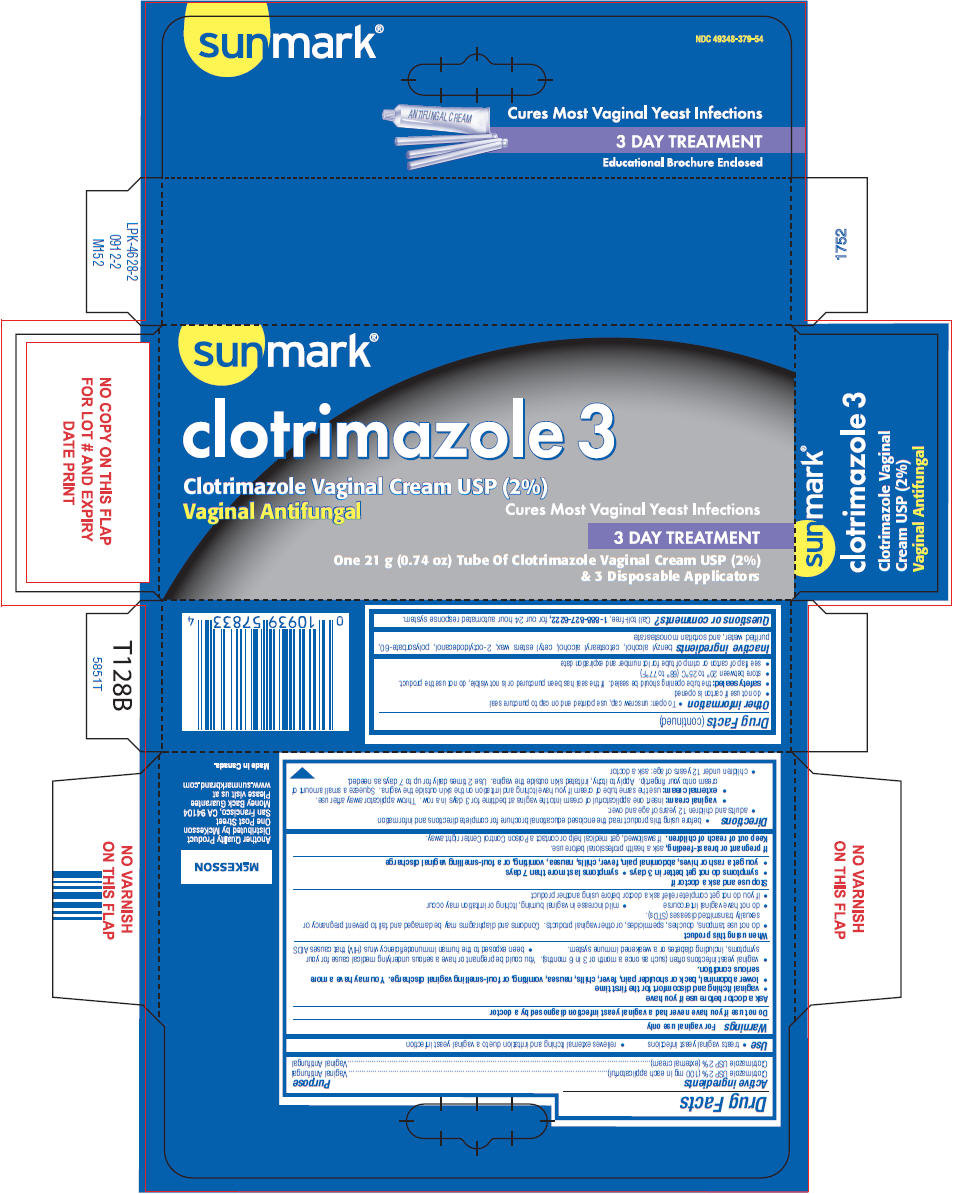
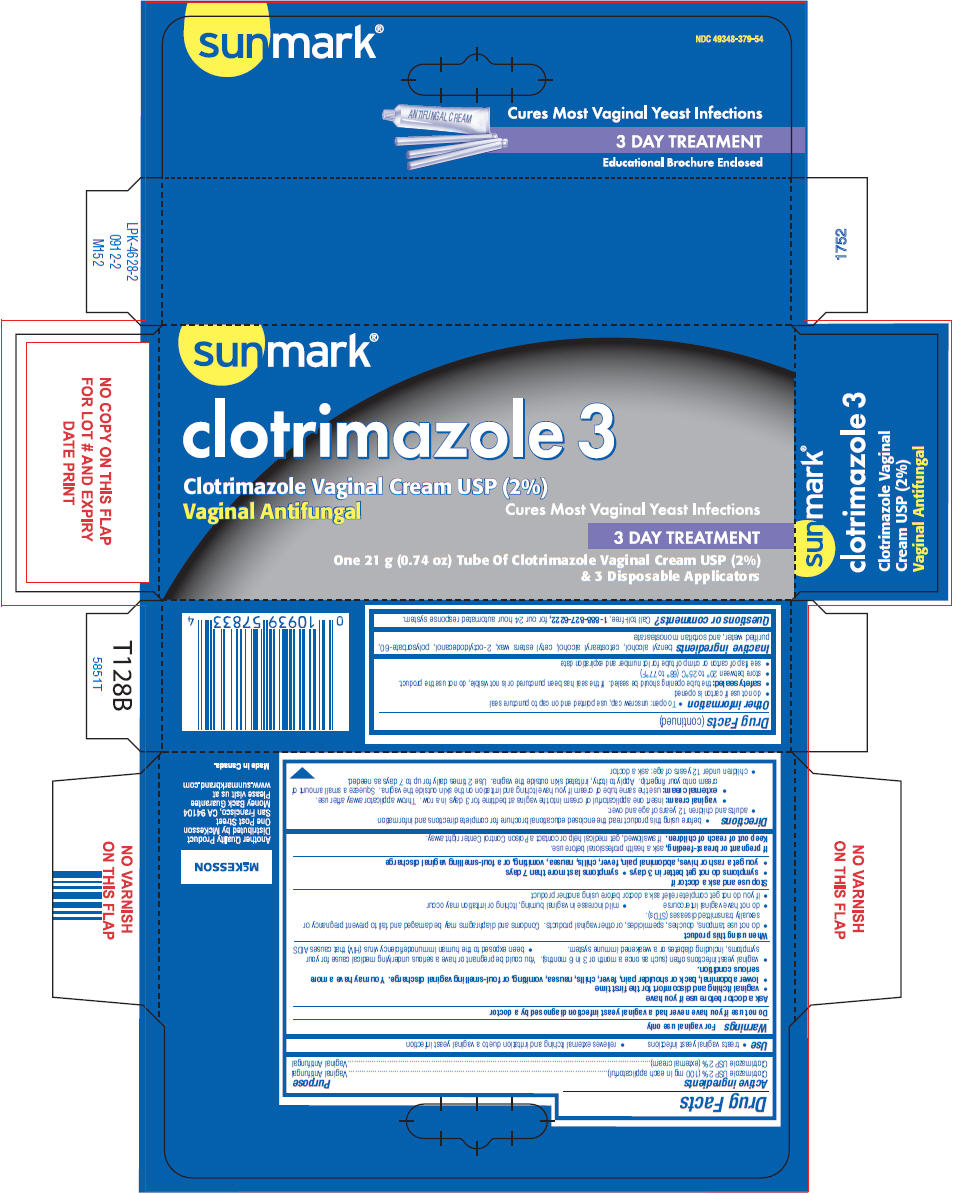
Label: SUNMARK CLOTRIMAZOLE 3- clotrimazole cream
- NDC Code(s): 49348-379-54
- Packager: Strategic Sourcing Services LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 21, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Use
-
Warnings
For vaginal use only
Ask a doctor before use if you have
- vaginal itching and discomfort for the first time
- lower abdominal, back or shoulder pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge. You may have a more serious condition.
- vaginal yeast infections often (such as once a month or 3 in 6 months). You could be pregnant or have a serious underlying medical cause for your symptoms, including diabetes or a weakened immune system.
- been exposed to the human immunodeficiency virus (HIV) that causes AIDS
When using this product
- do not use tampons, douches, spermicides, or other vaginal products. Condoms and diaphragms may be damaged and fail to prevent pregnancy or sexually transmitted diseases (STDs).
- do not have vaginal intercourse
- mild increase in vaginal burning, itching or irritation may occur
- if you do not get complete relief ask a doctor before using another product
-
Directions
- before using this product read the enclosed educational brochure for complete directions and information
- adults and children 12 years of age and over:
- vaginal cream: insert one applicatorful of cream into the vagina at bedtime for 3 days in a row. Throw applicator away after use.
- external cream: use the same tube of cream if you have itching and irritation on the skin outside the vagina. Squeeze a small amount of cream onto your fingertip. Apply to itchy, irritated skin outside the vagina. Use 2 times daily for up to 7 days as needed.
- children under 12 years of age: ask a doctor
-
Other information
- To open: unscrew cap, use pointed end on cap to puncture seal
- do not use if carton is opened
- safety sealed: the tube opening should be sealed. If the seal has been punctured or is not visible, do not use the product.
- store between 20° to 25°C (68° to 77°F)
- see flap of carton or crimp of tube for lot number and expiration date
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 21 g Tube Carton
-
INGREDIENTS AND APPEARANCE
SUNMARK CLOTRIMAZOLE 3
clotrimazole creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49348-379 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Clotrimazole (UNII: G07GZ97H65) (Clotrimazole - UNII:G07GZ97H65) Clotrimazole 2 g in 100 g Inactive Ingredients Ingredient Name Strength benzyl alcohol (UNII: LKG8494WBH) cetostearyl alcohol (UNII: 2DMT128M1S) cetyl esters wax (UNII: D072FFP9GU) octyldodecanol (UNII: 461N1O614Y) polysorbate 60 (UNII: CAL22UVI4M) water (UNII: 059QF0KO0R) sorbitan monostearate (UNII: NVZ4I0H58X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49348-379-54 1 in 1 CARTON 02/12/2013 1 21 g in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021143 04/12/2000 Labeler - Strategic Sourcing Services LLC (116956644) Registrant - Taro Pharmaceuticals U.S.A., Inc. (145186370) Establishment Name Address ID/FEI Business Operations Taro Pharmaceuticals Inc. 206263295 MANUFACTURE(49348-379)