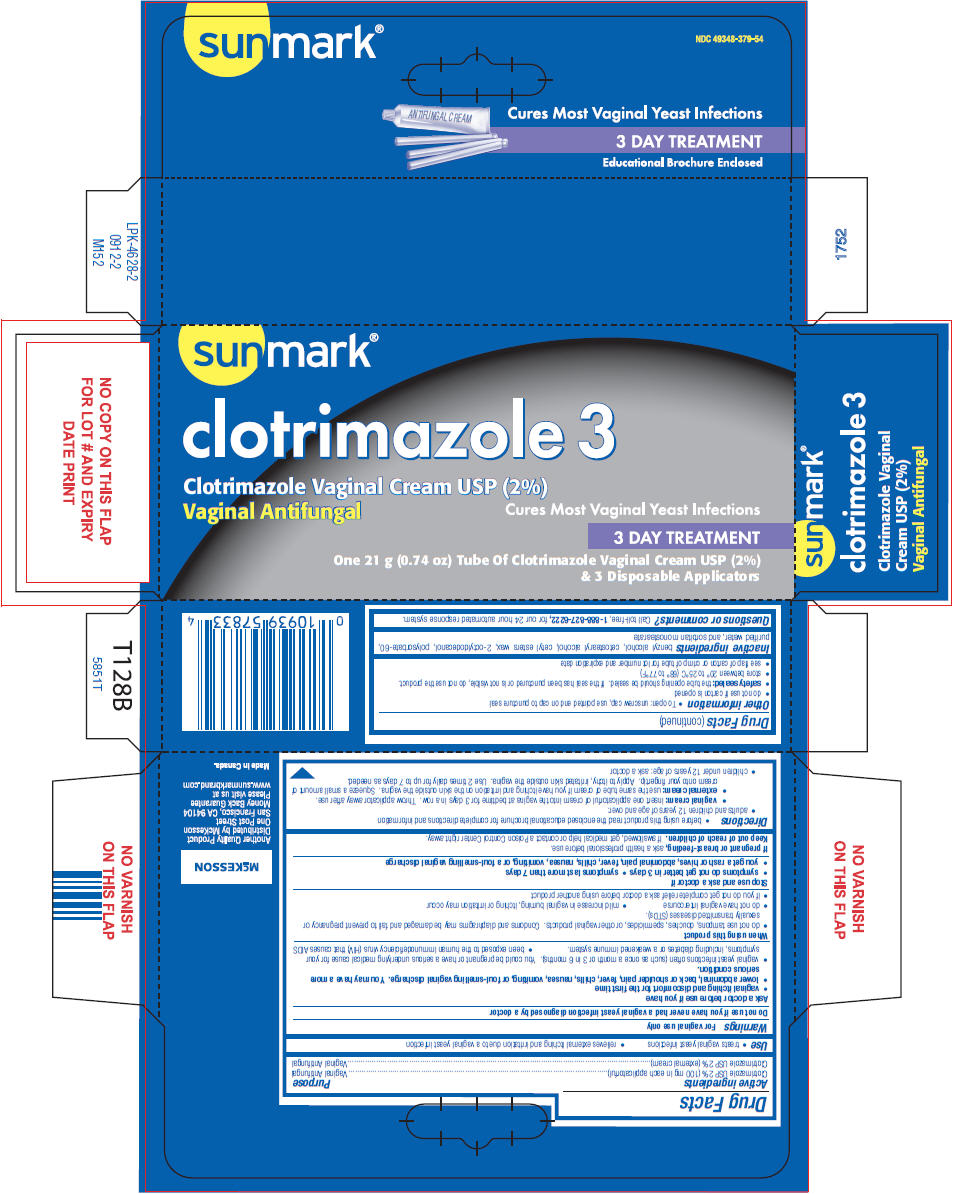
Use
- treats vaginal yeast infections
- relieves external itching and irritation due to a vaginal yeast infection
Warnings
For vaginal use only
Ask a doctor before use if you have
- vaginal itching and discomfort for the first time
- lower abdominal, back or shoulder pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge. You may have a more serious condition.
- vaginal yeast infections often (such as once a month or 3 in 6 months). You could be pregnant or have a serious underlying medical cause for your symptoms, including diabetes or a weakened immune system.
- been exposed to the human immunodeficiency virus (HIV) that causes AIDS
When using this product
- do not use tampons, douches, spermicides, or other vaginal products. Condoms and diaphragms may be damaged and fail to prevent pregnancy or sexually transmitted diseases (STDs).
- do not have vaginal intercourse
- mild increase in vaginal burning, itching or irritation may occur
- if you do not get complete relief ask a doctor before using another product
Directions
- before using this product read the enclosed educational brochure for complete directions and information
- adults and children 12 years of age and over:
- vaginal cream: insert one applicatorful of cream into the vagina at bedtime for 3 days in a row. Throw applicator away after use.
- external cream: use the same tube of cream if you have itching and irritation on the skin outside the vagina. Squeeze a small amount of cream onto your fingertip. Apply to itchy, irritated skin outside the vagina. Use 2 times daily for up to 7 days as needed.
- children under 12 years of age: ask a doctor
Other information
- To open: unscrew cap, use pointed end on cap to puncture seal
- do not use if carton is opened
- safety sealed: the tube opening should be sealed. If the seal has been punctured or is not visible, do not use the product.
- store between 20° to 25°C (68° to 77°F)
- see flap of carton or crimp of tube for lot number and expiration date