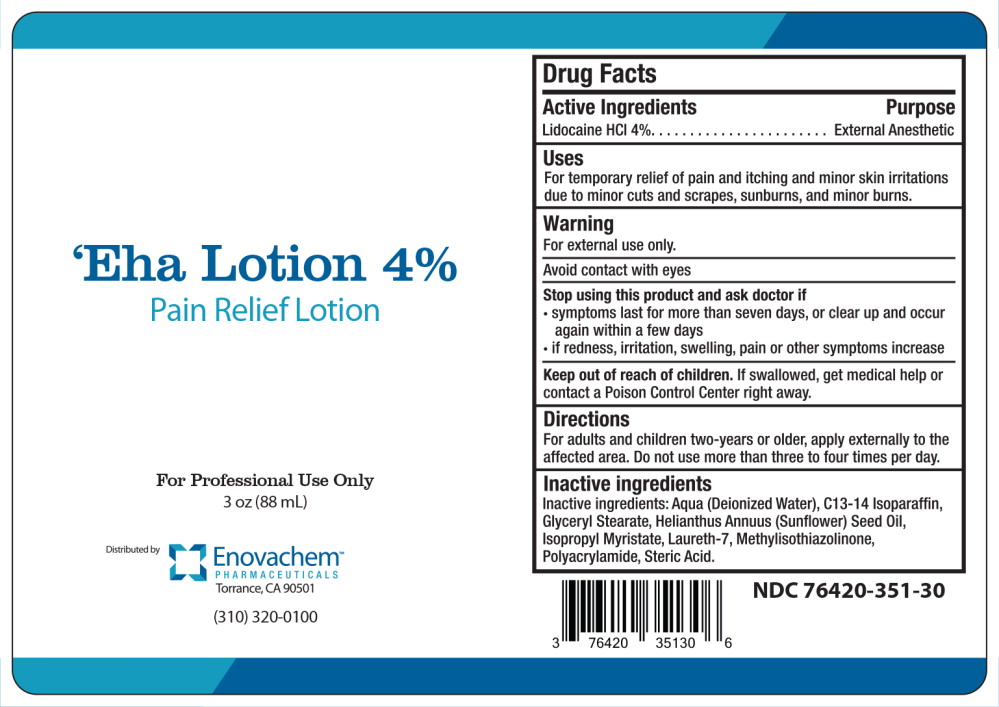
Label: EHA- lidocaine hydrochloride lotion
- NDC Code(s): 76420-351-30
- Packager: Asclemed USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 1, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses
- Warning
- Directions
- Inactive ingredients
- Principal Display Panel - 88 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
EHA
lidocaine hydrochloride lotionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76420-351 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 4 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) LAURETH-7 (UNII: Z95S6G8201) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76420-351-30 88 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/07/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/07/2016 Labeler - Asclemed USA, Inc. (059888437)