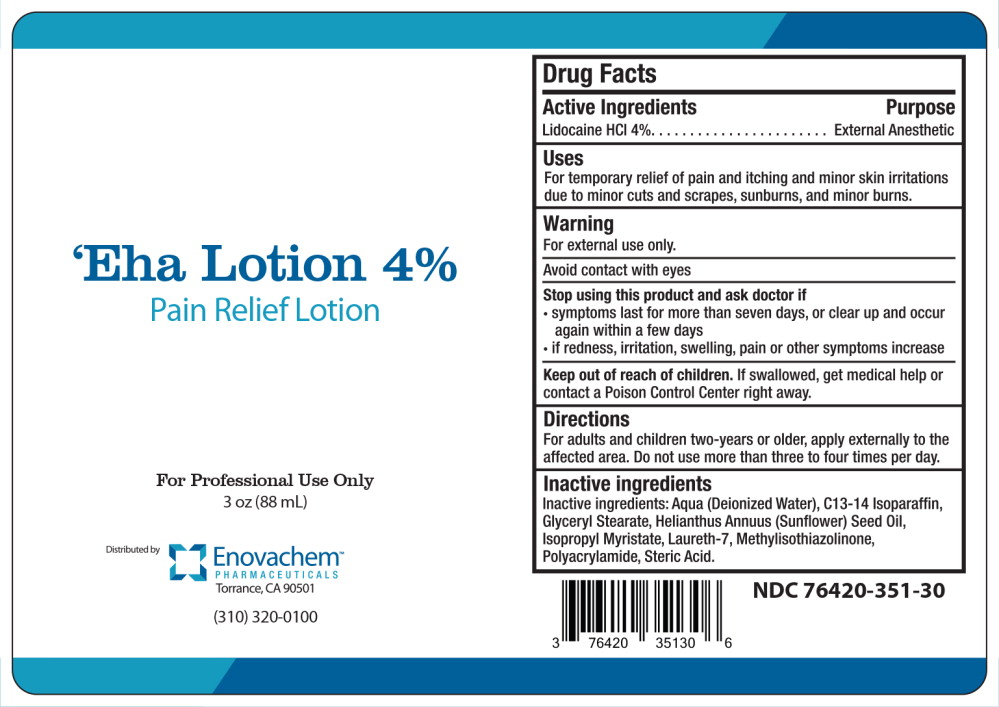
Active Ingredients
Lidocaine HCI 4%
Purpose
External Anesthetic
Uses
For temporary relief of pain and itching and minor skin irritations due to minor cuts and scrapes, sunburns, and minor burns.
Warning
For external use only.
Avoid contact with eyes
Stop using this product and ask doctor if
- symptoms last for more than seven days, or clear up and occur again within a few days
- if redness, irritation, swelling, pain or other symptoms increase
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
For adults and children two-years or older, apply externally to the affected area. Do not use more than three to four times per day.
Inactive ingredients
Inactive ingredients: Aqua (Deionized Water), C13-14 Isoparaffin, Glyceryl Stearate, Helianthus Annuus (Sunflower) Seed Oil, Isopropyl Myristate, Laureth-7, M Polyacrylamide, Steric Acid.
Principal Display Panel - 88 mL Bottle Label
‘Eha Lotion 4%
Pain Relief Lotion
For Professional Use Only
3 OZ (88 mL)
Distributed by
Enovachem
™
PHARMACEUTICALS
Torrance, CA 90501
(310) 320-0100
Asclemed USA, Inc.