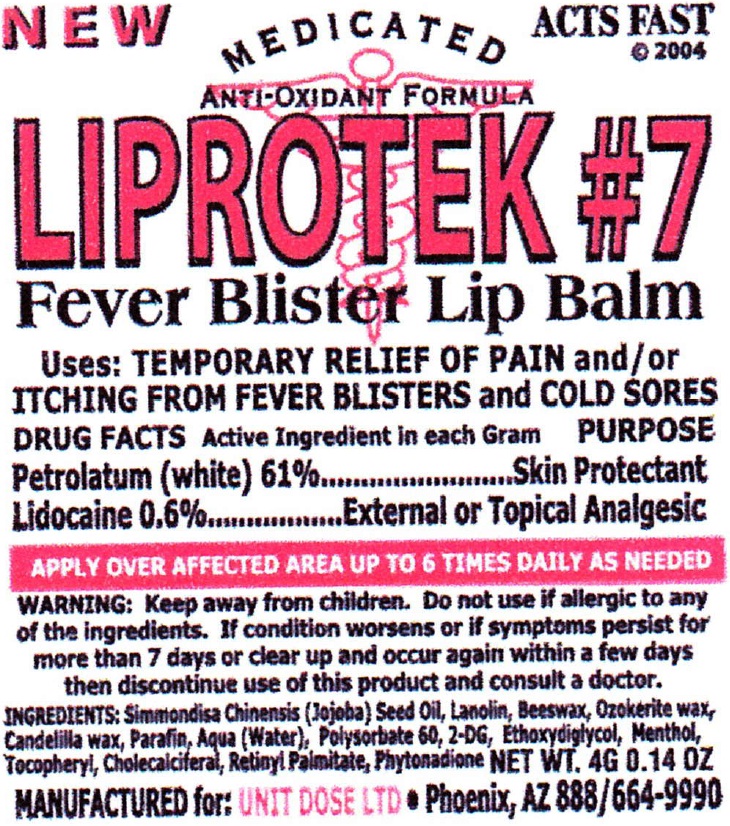
Label: LIPROTEK- petrolatum, lidocaine ointment
- NDC Code(s): 67194-017-01
- Packager: Unit Dose, Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Uses:
- DRUG FACTS
- Active Ingredient
- Uses:
- WARNING:
- INGREDIENTS:
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
LIPROTEK
petrolatum, lidocaine ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67194-017 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 610 mg in 1 g LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 6 mg in 1 g Inactive Ingredients Ingredient Name Strength JOJOBA OIL (UNII: 724GKU717M) LANOLIN (UNII: 7EV65EAW6H) YELLOW WAX (UNII: 2ZA36H0S2V) CERESIN (UNII: Q1LS2UJO3A) CANDELILLA WAX (UNII: WL0328HX19) PARAFFIN (UNII: I9O0E3H2ZE) WATER (UNII: 059QF0KO0R) POLYSORBATE 60 (UNII: CAL22UVI4M) 2-DEOXYGLUCOSE (UNII: 9G2MP84A8W) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) MENTHOL (UNII: L7T10EIP3A) CHOLECALCIFEROL (UNII: 1C6V77QF41) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) PHYTONADIONE (UNII: A034SE7857) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67194-017-01 4 g in 1 JAR; Type 0: Not a Combination Product 01/21/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/21/2016 Labeler - Unit Dose, Ltd. (119080393)