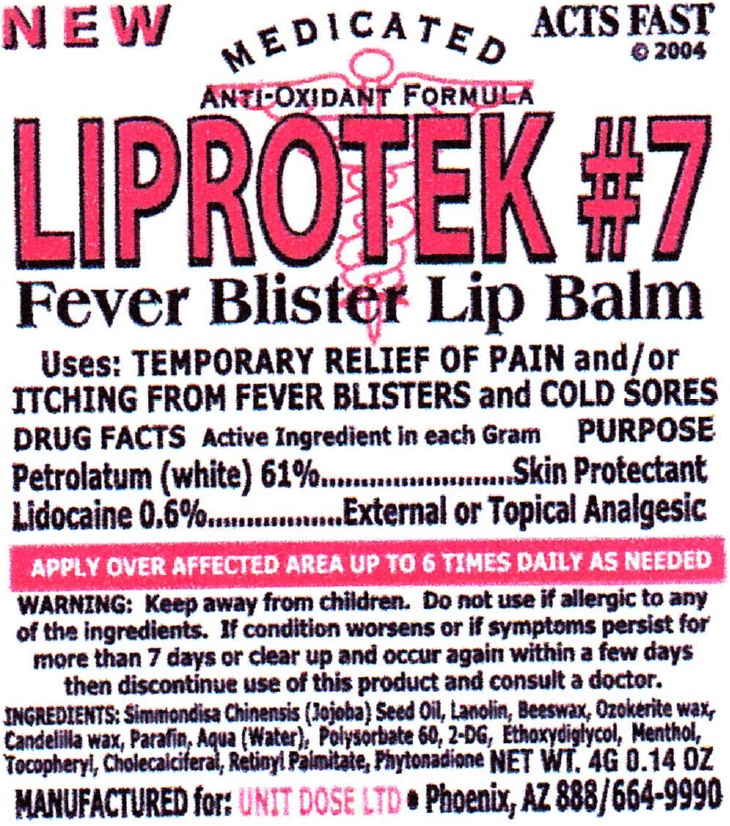
Uses:
TEMPORARY RELIEF OF PAIN and/or ITCHING FROM FEVER BLISTERS and COLD SORES
Active Ingredient
Petrolatum (white) 61%
Lidocaine 0.6%
PURPOSE
Skin Protectant
External or Topical Analgesic
Uses:
TEMPORARY RELIEF OF PAIN AND/OR ITCHING FROM FEVER BLISTERS AND COLD SORES
WARNING:
Do not use
if allergic tp any of the ingredients. If condition worsens or if symptoms persist for more than 7 days or clear up and occur again within a few days then discontinue use of this product and cunsult a doctor.
INGREDIENTS:
Simmondisa Chinensis (Jojoba) Seed Oil, Lanolin, Beeswax, Ozokerite wax, Candelilla wax, Parafin, Aqua (Water), Polysorbate 60, 2-DG, Ethoxydiglycol, Menthol, Tocopheryl, Cholecalciferal, Retinyl Palmitate, Phytonadione
Package Labeling:
Unit Dose, Ltd.