Label: SPRINSOL- chlorpheniramine maleate tablet
- NDC Code(s): 51467-101-01, 51467-101-02
- Packager: FORTUNE PHARMACAL COMPANY LIMITED
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 22, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR/PHARMACIST
- WHEN USING
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
-
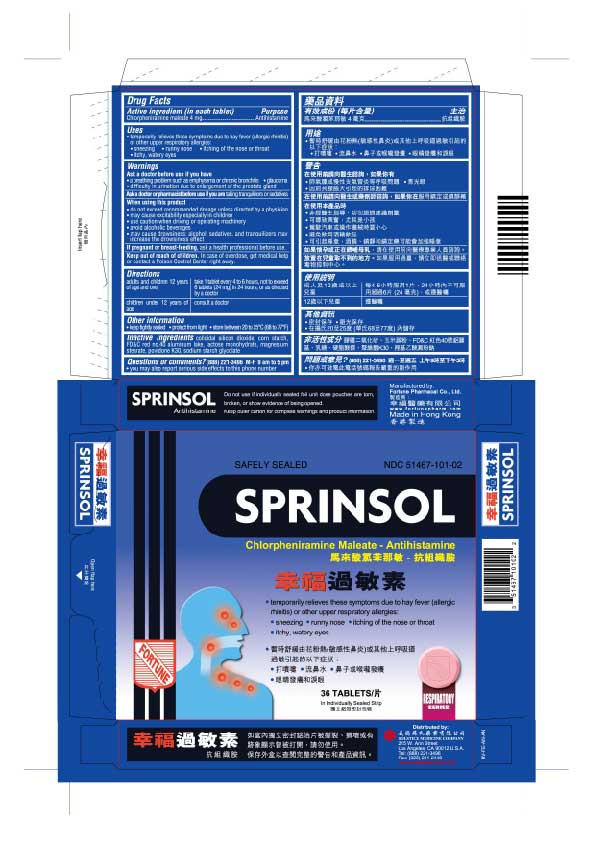
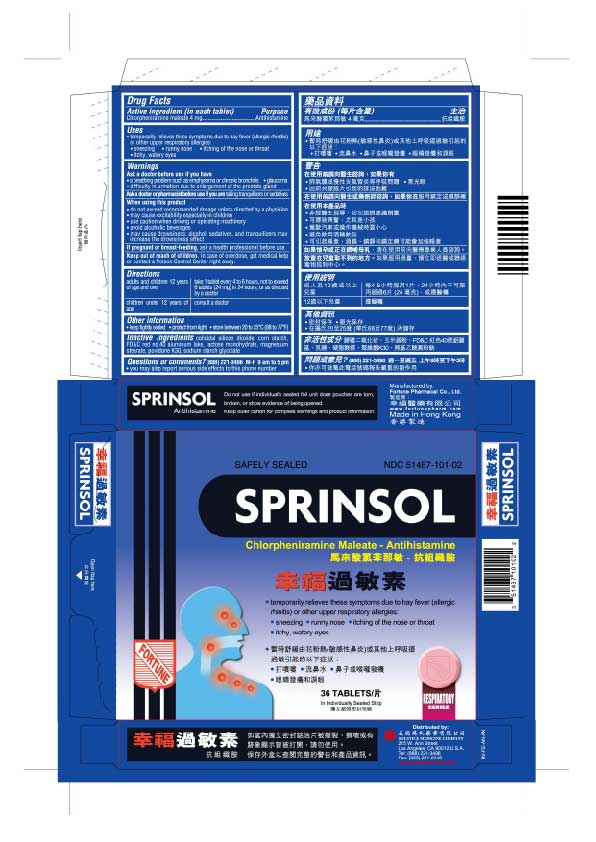
PRINCIPAL DISPLAY PANEL
Sprinsol
Safely Sealed
NDC 51467-101-02
Chlorpheniramine maleate - Antihistamine
temporarily relieves these symptoms due to hay fever (allergic rhinitis) or other upper respiratory allergies:
sneezing
runny nose
itching of the nose or throat
itchy, watery eyes36 Tablets
In Individually Sealed Strip
Respiratory Series
-
INGREDIENTS AND APPEARANCE
SPRINSOL
chlorpheniramine maleate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51467-101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 4 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) FD&C RED NO. 40 (UNII: WZB9127XOA) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE K30 (UNII: U725QWY32X) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color red Score no score Shape ROUND Size 10mm Flavor Imprint Code FORTUNE Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51467-101-01 2 in 1 BOX 09/22/2017 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:51467-101-02 3 in 1 BOX 09/22/2017 2 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 09/22/2017 Labeler - FORTUNE PHARMACAL COMPANY LIMITED (686280561) Establishment Name Address ID/FEI Business Operations FORTUNE PHARMACAL COMPANY LIMITED 686280561 manufacture(51467-101)