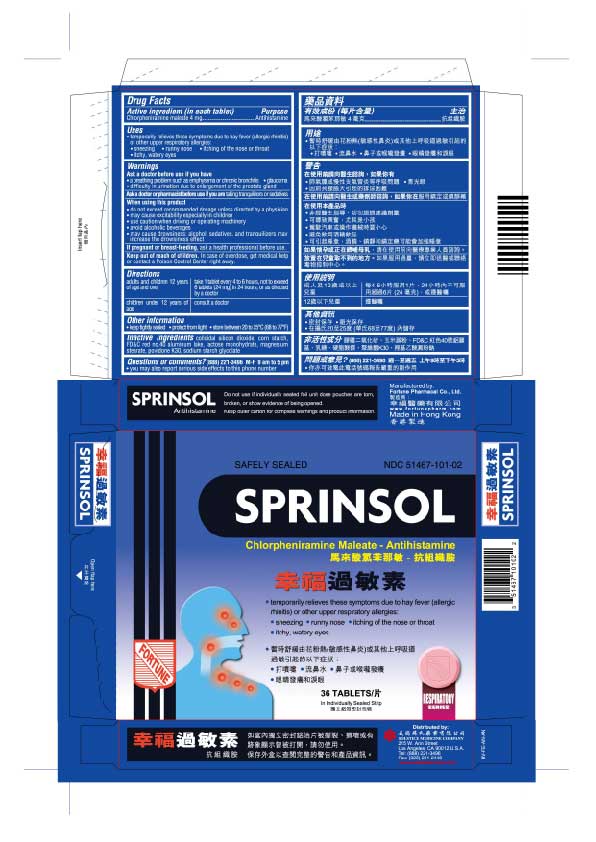
Uses
temporarily relieves these symptoms due to hay fever (allergic rhinitis) or other upper respiratory allergies:
sneezing
runny nose
itching of the nose or throat
itchy, watery eyes
Warnings
Ask a doctor before use if you have
a breathing problem such as emphysema or chronic bronchitis
glaucoma
difficulty in urination due to enlargement of the prostate gland
When using this product
do not exceed recommended dosage unless directed by a physician
may cause excitability especially in children
use caution when driving or operating machinery
avoid alcoholic beverages
may cause drowsiness; alcohol, sedatives, and tranquilizers may increase the drowsiness effect
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away
Directions
adults and children 12 years of age and over: take 1 tablet every 4 to 6 hours, not to exceed 6 tablets (24 mg) in 24 hours, or as directed by a doctor
children under 12 years of age: consult a doctor
Inactive ingredients
colloidal silicon dioxide, corn starch, FD C red no. 40 aluminum lake, lactose monohydrate, magnesium stearate, povidone K30, sodium starch glycolate
Questions or comments? (888) 221-3496 M-F 9 am to 5 pm
you may also report serious side effects to this phone number
Sprinsol
Safely Sealed
NDC 51467-101-02
Chlorpheniramine maleate - Antihistamine
temporarily relieves these symptoms due to hay fever (allergic rhinitis) or other upper respiratory allergies:
sneezing
runny nose
itching of the nose or throat
itchy, watery eyes
36 Tablets
In Individually Sealed Strip
Respiratory Series