Label: Y LAX DR- bisacodyl tablet, sugar coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 68210-0001-2, 68210-0001-3, 68210-0001-4, 68210-0001-5 - Packager: SPIRIT PHARMACEUTICALS,LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 21, 2010
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- Stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that persists over a period of 2 weeks
When using this product
- do not chew or crush tablets
- do not take this product within 1 hour after taking an antacid or milk
- it may cause stomach discomfort,faintness, and cramps
- Direction
- Other information
-
Inactive ingredients
lactose,cornstarch,povidone (K-30),sodium startch glycolate,talc,magnesium stearate,methacrylic acid copolymer,polethylene glaycol,sodium hydroxide pellets, sucrose,acacia,gelatin,methylparaben, propylparaben, calcium sulphate dihydrate,titanium dioxide,D&C yellow #6 lake, FD & C yellow #10; pharmaceuticals glaze
-
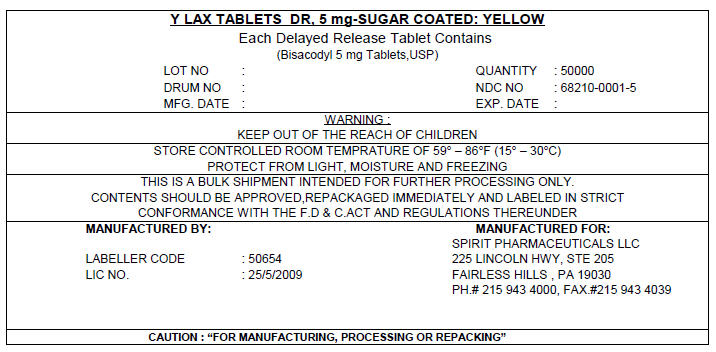
PRINCIPAL DISPLAY PANEL - 5 mg Shipping Label
Y LAX TABLETS DR, 5 mg-SUGAR COATED: YELLOW
Each Delayed Release Tablet Contains
(Bisacodyl 5 mg Tablets,USP)
LOT NO :
DRUM NO :
MFG. DATE :QUANTITY : 50000
NDC NO : 68210-0001-5
EXP. DATE :WARNING :
KEEP OUT OF THE REACH OF CHILDRENSTORE CONTROLLED ROOM TEMPRATURE OF 59° – 86°F (15° – 30°C)
PROTECT FROM LIGHT, MOISTURE AND FREEZINGTHIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY.
CONTENTS SHOULD BE APPROVED,REPACKAGED IMMEDIATELY AND LABELED IN STRICT
CONFORMANCE WITH THE F.D & C.ACT AND REGULATIONS THEREUNDERMANUFACTURED BY:
LABELLER CODE : 50654
LIC NO. : 25/5/2009MANUFACTURED FOR:
SPIRIT PHARMACEUTICALS LLC
225 LINCOLN HWY, STE 205
FAIRLESS HILLS , PA 19030
PH.# 215 943 4000, FAX.#215 943 4039CAUTION : "FOR MANUFACTURING, PROCESSING OR REPACKING"
-
INGREDIENTS AND APPEARANCE
Y LAX DR
bisacodyl tablet, sugar coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68210-0001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISACODYL (UNII: 10X0709Y6I) (BISACODYL - UNII:10X0709Y6I) BISACODYL 5 mg Inactive Ingredients Ingredient Name Strength LACTOSE (UNII: J2B2A4N98G) STARCH, CORN (UNII: O8232NY3SJ) POVIDONE K30 (UNII: U725QWY32X) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) SUCROSE (UNII: C151H8M554) ACACIA (UNII: 5C5403N26O) METHYLPARABEN (UNII: A2I8C7HI9T) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) ALUMINUM OXIDE (UNII: LMI26O6933) Product Characteristics Color YELLOW (ORANGE YELLOW) Score no score Shape ROUND Size 4mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68210-0001-5 1 in 1 DRUM 1 50000 in 1 BAG 2 NDC:68210-0001-2 1 in 1 DRUM 2 250000 in 1 BAG 3 NDC:68210-0001-3 1 in 1 DRUM 3 300000 in 1 BAG 4 NDC:68210-0001-4 1 in 1 DRUM 4 350000 in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 05/01/2010 Labeler - SPIRIT PHARMACEUTICALS,LLC (179621011) Establishment Name Address ID/FEI Business Operations MISSION VIVACARE LIMITED 677604252 API MANUFACTURE, RECOVERY