Uses
- for relief of occasional constipation(irregularity)
- this product generally produces bowel movement in 6 to 12 hours
Warnings
Ask a doctor before use if you have
- Stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that persists over a period of 2 weeks
When using this product
- do not chew or crush tablets
- do not take this product within 1 hour after taking an antacid or milk
- it may cause stomach discomfort,faintness, and cramps
Direction
take with a glass of water
- Do not take more than three tablets daily
| adults and children 12 years of age and older | - take 1 to 3 tablets in a single dose once daily |
| children 6 to under 12 years of age | - take 1 tablet once daily |
| children under 6 years of age | - ask a doctor |
Inactive ingredients
lactose,cornstarch,povidone (K-30),sodium startch glycolate,talc,magnesium stearate,methacrylic acid copolymer,polethylene glaycol,sodium hydroxide pellets, sucrose,acacia,gelatin,methylparaben, propylparaben, calcium sulphate dihydrate,titanium dioxide,D&C yellow #6 lake, FD & C yellow #10; pharmaceuticals glaze
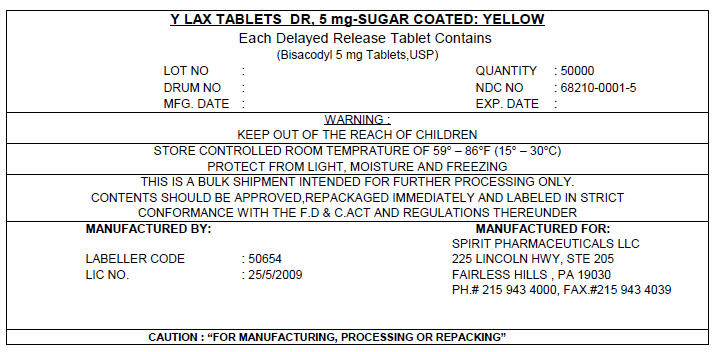
PRINCIPAL DISPLAY PANEL - 5 mg Shipping Label
Y LAX TABLETS DR, 5 mg-SUGAR COATED: YELLOW
Each Delayed Release Tablet Contains
(Bisacodyl 5 mg Tablets,USP)
LOT NO :
DRUM NO :
MFG. DATE :
QUANTITY : 50000
NDC NO : 68210-0001-5
EXP. DATE :
WARNING :
KEEP OUT OF THE REACH OF CHILDREN
STORE CONTROLLED ROOM TEMPRATURE OF 59° – 86°F (15° – 30°C)
PROTECT FROM LIGHT, MOISTURE AND FREEZING
THIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY.
CONTENTS SHOULD BE APPROVED,REPACKAGED IMMEDIATELY AND LABELED IN STRICT
CONFORMANCE WITH THE F.D & C.ACT AND REGULATIONS THEREUNDER
MANUFACTURED BY:
LABELLER CODE : 50654
LIC NO. : 25/5/2009
MANUFACTURED FOR:
SPIRIT PHARMACEUTICALS LLC
225 LINCOLN HWY, STE 205
FAIRLESS HILLS , PA 19030
PH.# 215 943 4000, FAX.#215 943 4039
CAUTION : "FOR MANUFACTURING, PROCESSING OR REPACKING"