Label: ANTISEPTIC BANDAGES- benzalkonium chloride dressing
- NDC Code(s): 28691-0010-5, 28691-0010-6, 28691-0010-7
- Packager: Pharmaplast SAE
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 7, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
- Inactive Ingredients
-
SPL UNCLASSIFIED SECTION
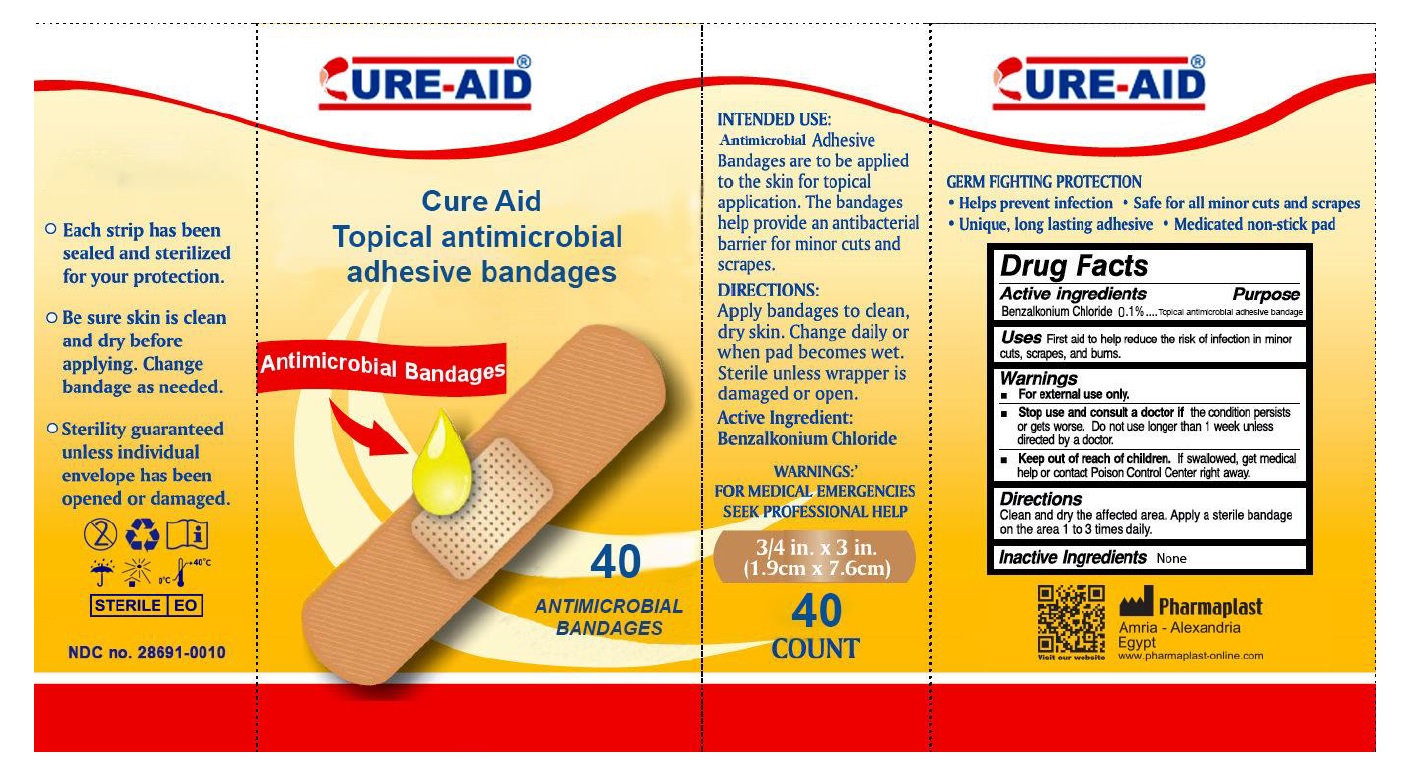
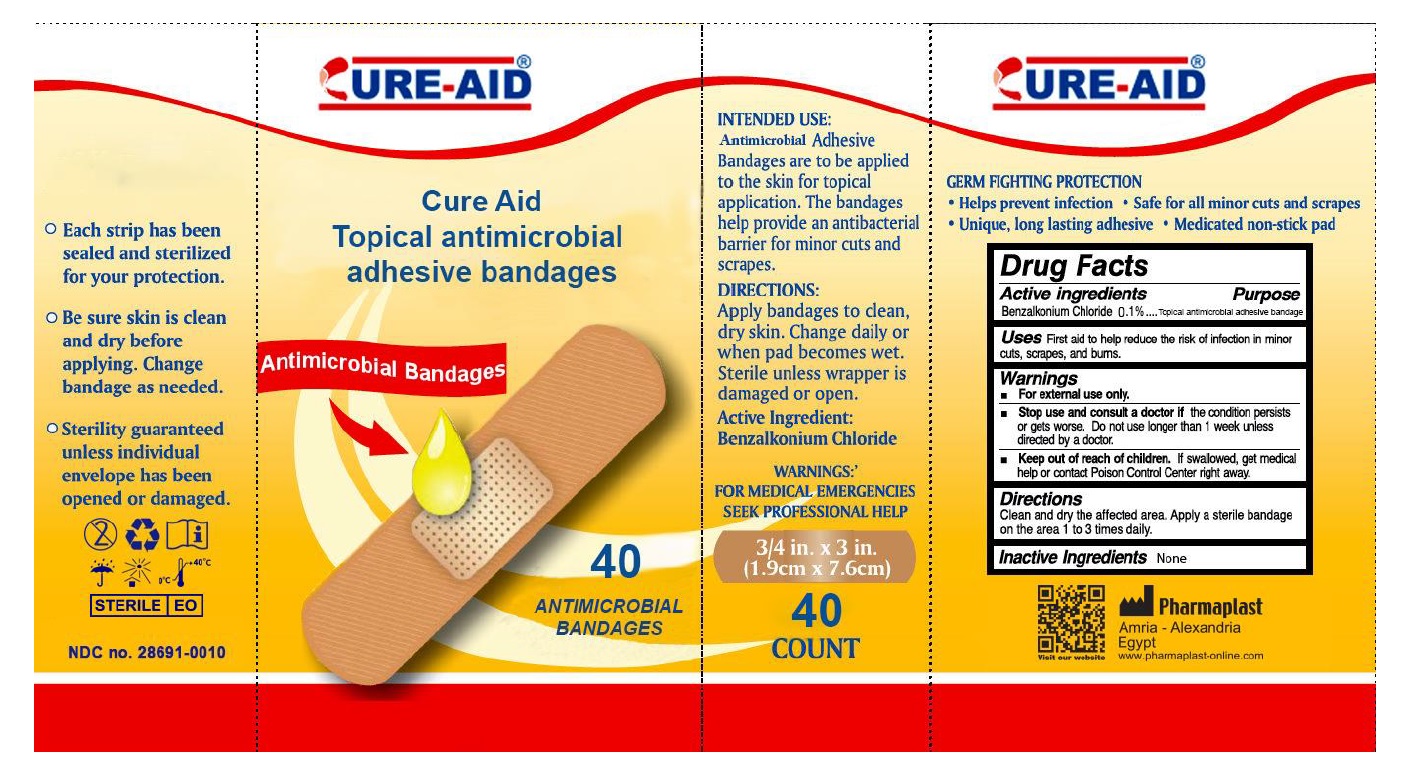
CURE-AID®
INTENDED USE:
Antimicrobial Adhesive Bandages are to be applied to the skin for topical application. The bandages help provide an antibacterial barrier for minor cuts and scrapes.DIRECTIONS:
Apply bandages to clean, dry skin. Change daily or when pad becomes wet. Sterile unless wrapper is damaged or open.WARNINGS:
FOR MEDICAL EMERGENCIES SEEK PROFESSIONAL HELPGERM FIGHTING PROTECTION
• Help prevent infection • Safe for all minor cuts and scrapes • Unique, long lasting adhesive • Medicated non-stick pad- Each strip has been sealed and sterilized for your protection.
- Be sure skin is clean and dry before applying. Change bandage as needed.
- Sterility guaranteed unless individual envelope has been opened or damaged.
Pharmaplast
Amria – Alexandria
Egypt
www.Pharmaplast-online.com - Packaging
-
INGREDIENTS AND APPEARANCE
ANTISEPTIC BANDAGES
benzalkonium chloride dressingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:28691-0010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.1 mg in 100 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity Does not contain NATURAL LATEX RUBBER (UNII: 2LQ0UUW8IN) 0 mg in 100 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:28691-0010-5 20 in 1 BOX 10/07/2016 1 0.033 mg in 1 PATCH; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug 2 NDC:28691-0010-6 30 in 1 BOX 10/07/2016 2 0.033 mg in 1 PATCH; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug 3 NDC:28691-0010-7 40 in 1 BOX 10/07/2016 3 0.033 mg in 1 PATCH; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 10/07/2016 Labeler - Pharmaplast SAE (644773319) Establishment Name Address ID/FEI Business Operations Pharmaplast SAE 644773319 analysis(28691-0010) , label(28691-0010) , manufacture(28691-0010) , pack(28691-0010)