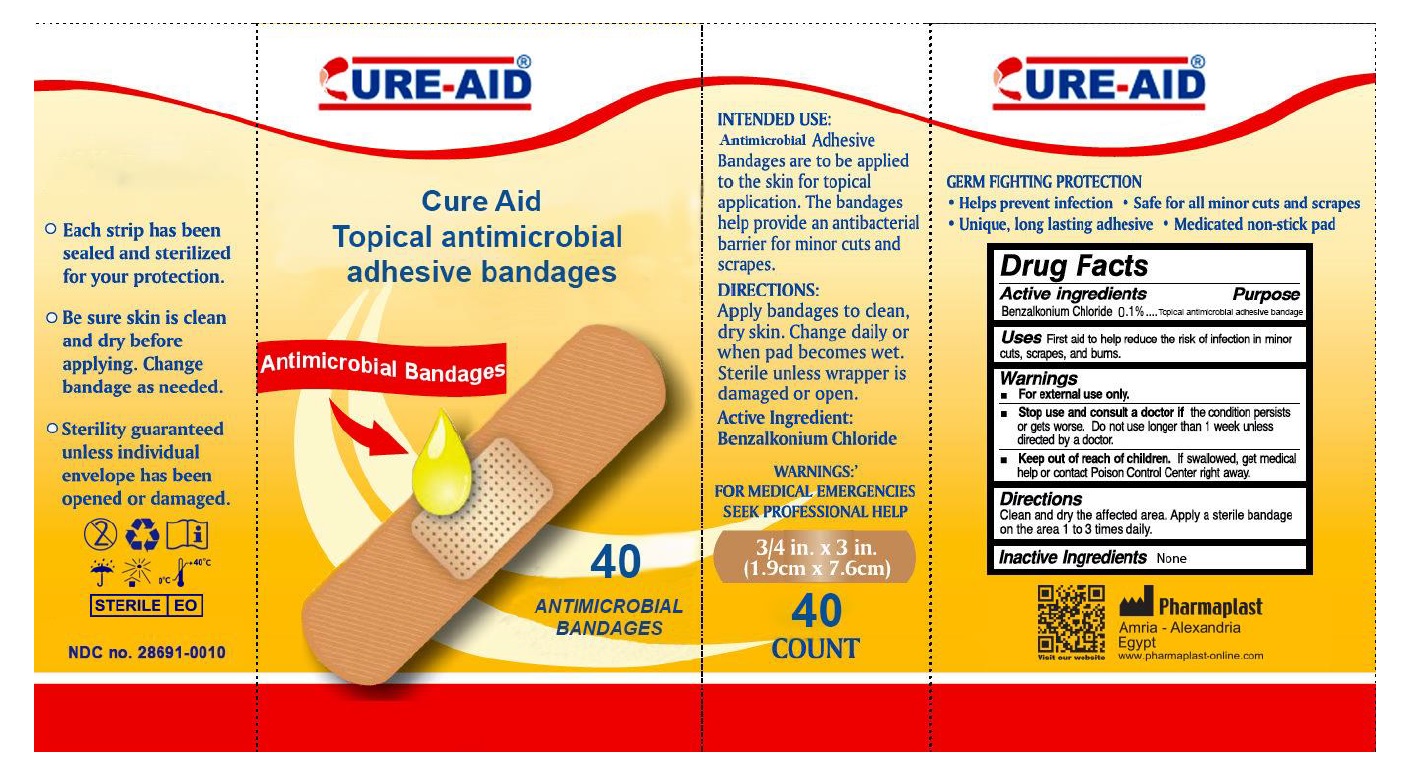
Warnings
- For external use only.
- Stop use and consult a doctor if the condition persists or gets worse. Do not use longer than 1 week unless directed by a doctor.
CURE-AID®
INTENDED USE:
Antimicrobial Adhesive Bandages are to be applied to the skin for topical application. The bandages help provide an antibacterial barrier for minor cuts and scrapes.
DIRECTIONS:
Apply bandages to clean, dry skin. Change daily or when pad becomes wet. Sterile unless wrapper is damaged or open.
WARNINGS:
FOR MEDICAL EMERGENCIES SEEK PROFESSIONAL HELP
GERM FIGHTING PROTECTION
• Help prevent infection • Safe for all minor cuts and scrapes • Unique, long lasting adhesive • Medicated non-stick pad
- Each strip has been sealed and sterilized for your protection.
- Be sure skin is clean and dry before applying. Change bandage as needed.
- Sterility guaranteed unless individual envelope has been opened or damaged.
Pharmaplast
Amria – Alexandria
Egypt
www.Pharmaplast-online.com