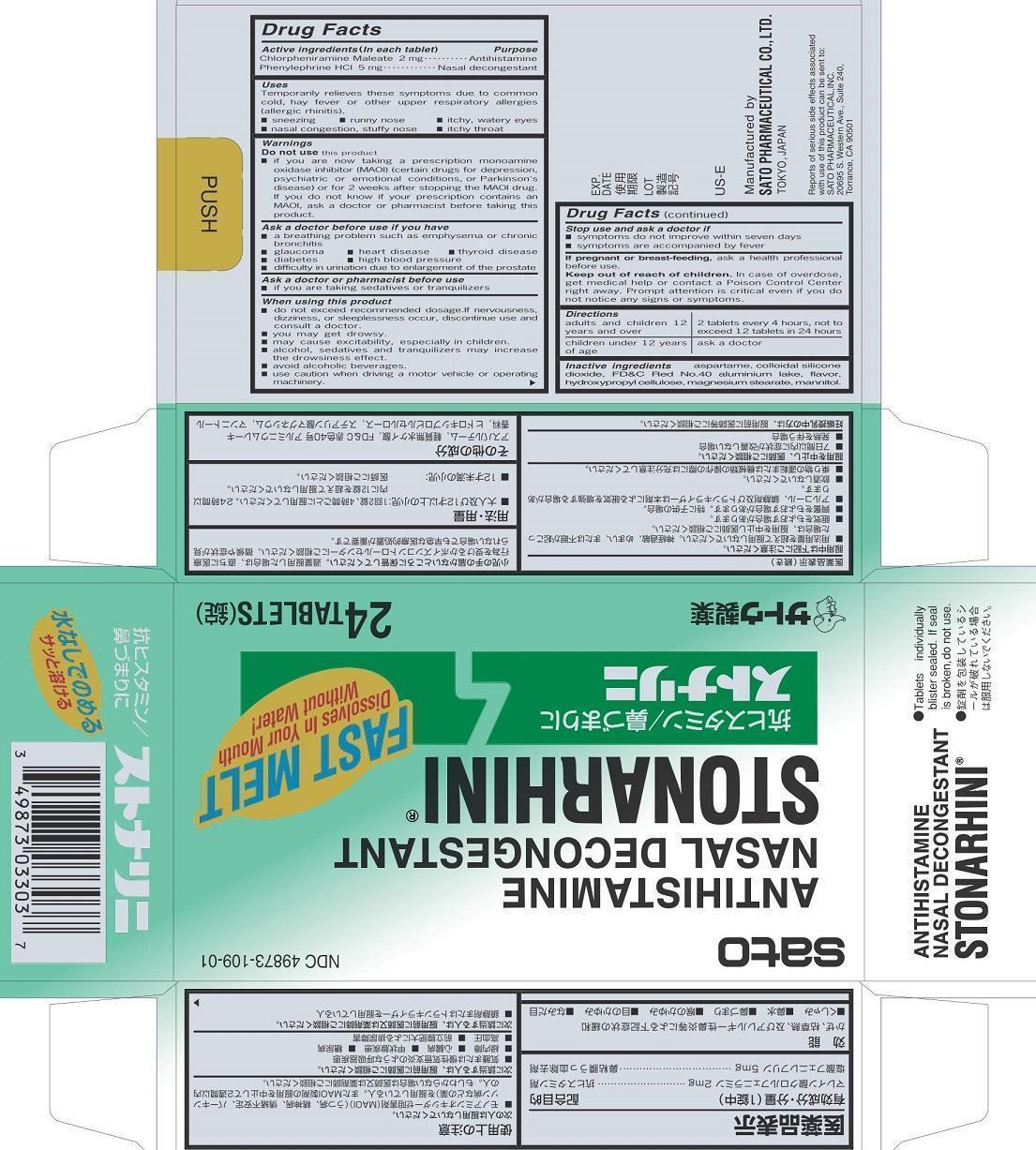
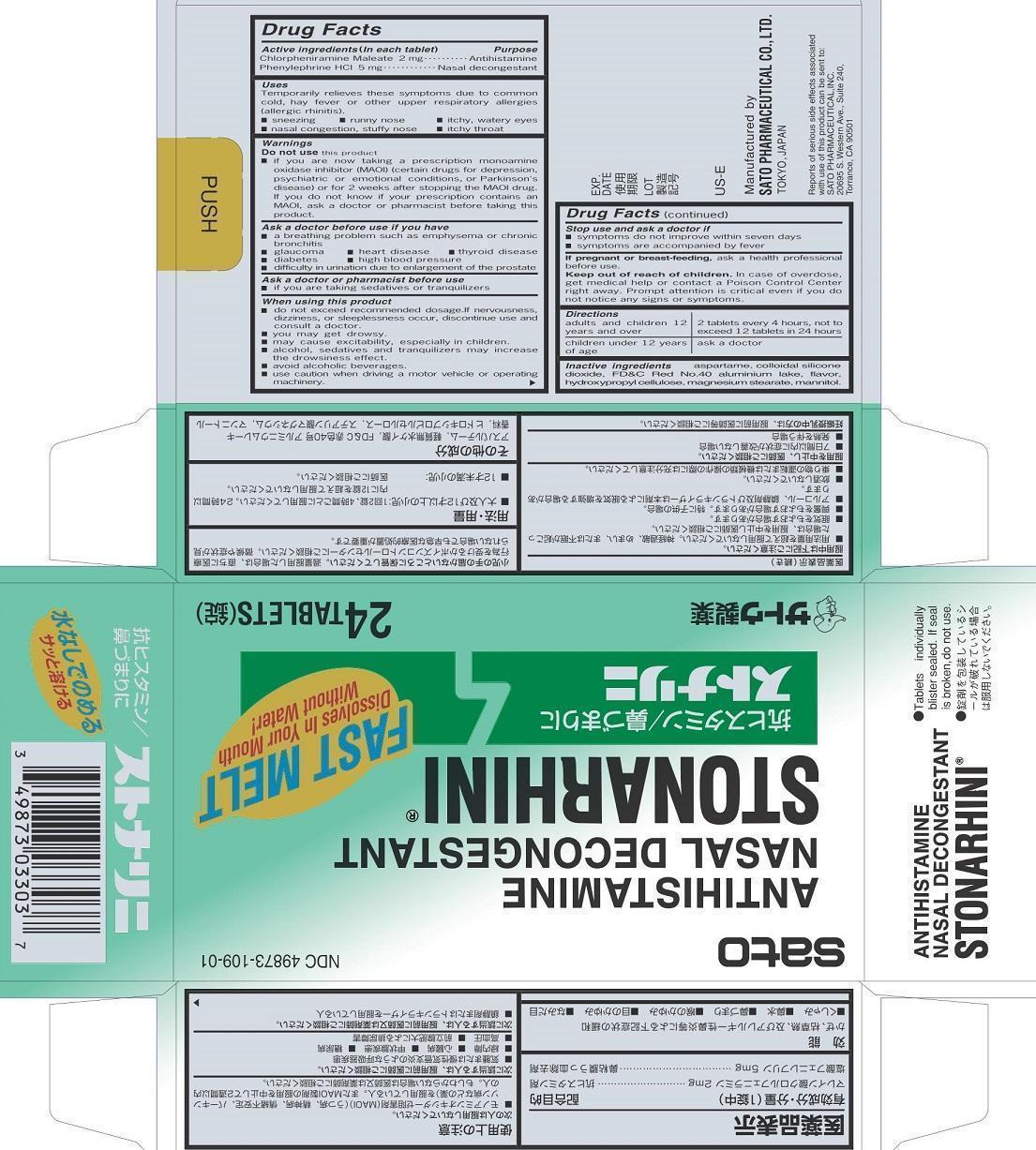
Label: STONARHINI- chlorpheniramine maleate, phenylephrine tablet
- NDC Code(s): 49873-109-01
- Packager: Sato Pharmaceutical Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
Warnings
Enter section text here
Do not use this product
■ if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease) or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription contains an MAOI, ask a doctor or pharmacist before taking this priduct.Ask a doctor before use if you have
■ a breathing problem such as emphysema or chronic bronchitis
■ glaucoma ■ heart disease ■ thyroid disease
■ diabetes ■ high blood pressure
■difficulty in urination due to enlargement of the prostateWhen using this product
■ do not exceed recommended dosage. If nervousness, dizziness, or sleeplessness occur, discontinue use and consult a doctor.
■ you may get drowsy.
■ may cause excitability, especially in children.
■ alcohol, sedatives and tranquilizers may increase the drowsiness effect.
■ avoid alcoholic beverages.
■ use caution when driving a motor vehicle or operating machinery. - PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
STONARHINI
chlorpheniramine maleate, phenylephrine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49873-109 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 2 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C RED NO. 40 (UNII: WZB9127XOA) HYDROXYPROPYL CELLULOSE (TYPE EL) (UNII: 8VAB711C5E) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) ALUMINUM OXIDE (UNII: LMI26O6933) Product Characteristics Color red (pale red) Score no score Shape ROUND Size 10mm Flavor MENTHOL Imprint Code S10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49873-109-01 4 in 1 CARTON 11/16/2001 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/16/2001 Labeler - Sato Pharmaceutical Co., Ltd. (690575642) Establishment Name Address ID/FEI Business Operations Sato Pharmaceutical Co., Ltd. 715699133 manufacture(49873-109) , label(49873-109) , pack(49873-109)