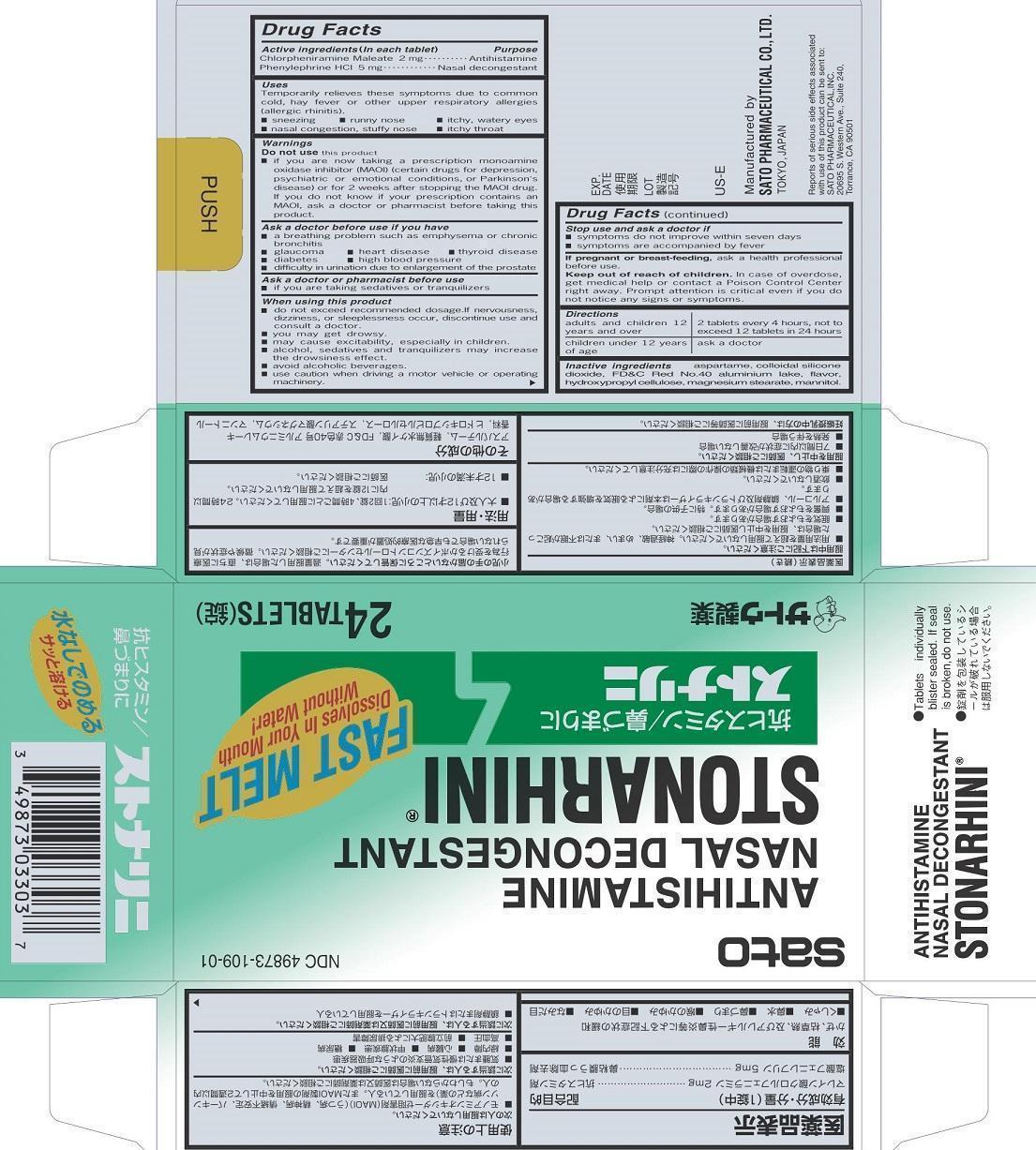
Uses
Temporarily relieves these symptoms due to common cold, hay fever or other upper respiratory allergies (allergic rhinitis).
■ sneezing ■ runny nose ■ itchy, watery eyes
■ nasal congestion, stuffy nose ■ itchy throat
Warnings
Enter section text here
Do not use this product
■ if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease) or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription contains an MAOI, ask a doctor or pharmacist before taking this priduct.
Ask a doctor before use if you have
■ a breathing problem such as emphysema or chronic bronchitis
■ glaucoma ■ heart disease ■ thyroid disease
■ diabetes ■ high blood pressure
■difficulty in urination due to enlargement of the prostate
When using this product
■ do not exceed recommended dosage. If nervousness, dizziness, or sleeplessness occur, discontinue use and consult a doctor.
■ you may get drowsy.
■ may cause excitability, especially in children.
■ alcohol, sedatives and tranquilizers may increase the drowsiness effect.
■ avoid alcoholic beverages.
■ use caution when driving a motor vehicle or operating machinery.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. Prompt attention is critical even if you do not notice any signs or symptoms.
Directions
adults and children 12 years and over: 2 tablets every 4 hours, not to exceed 12 tablets in 24 hours
children under 12 years of age: ask a doctor