Label: HELP I HAVE ALLERGIES- loratadine tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 49260-611-08, 49260-611-24 - Packager: Help Remedies, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 6, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
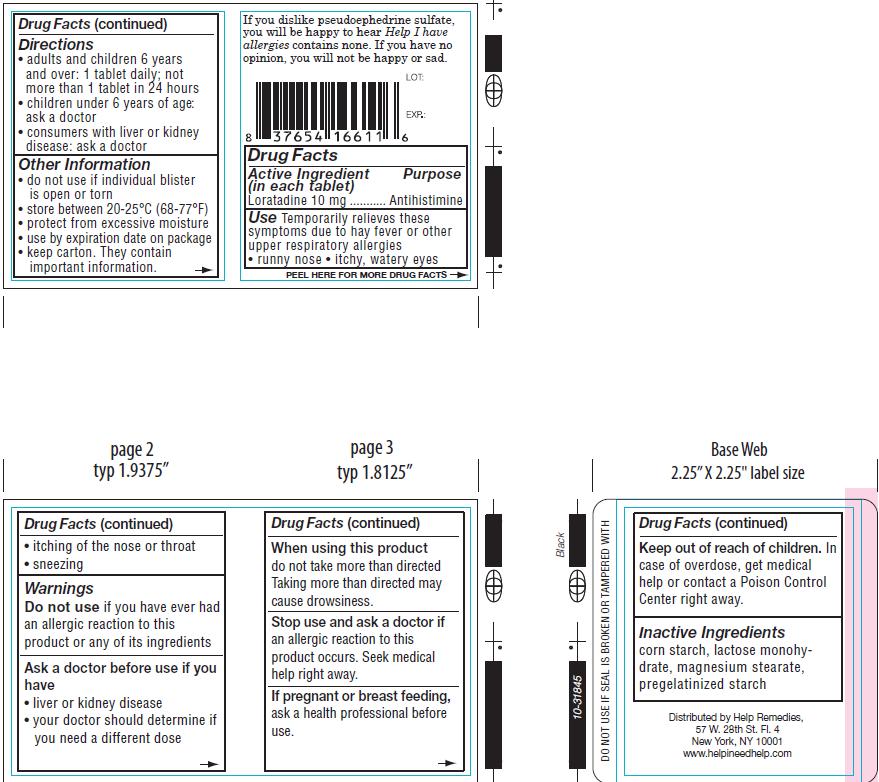
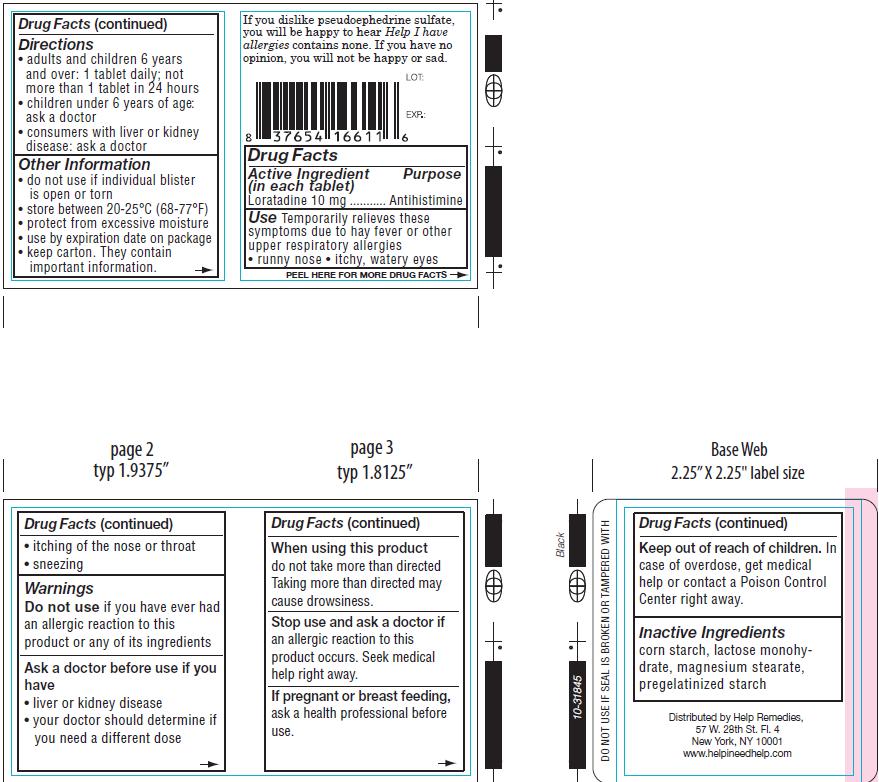
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings
Do not use ifyou have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- liver or kidney disease
- your doctor should determine if you need a different dose
cause drowsiness.
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical
help right away.
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HELP I HAVE ALLERGIES
loratadine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49260-611 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Loratadine (UNII: 7AJO3BO7QN) (Loratadine - UNII:7AJO3BO7QN) Loratadine 10 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white (white) Score no score Shape ROUND (round) Size 7mm Flavor Imprint Code RX526 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49260-611-24 24 in 1 BOTTLE 2 NDC:49260-611-08 8 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/01/2009 Labeler - Help Remedies, Inc. (050359682)