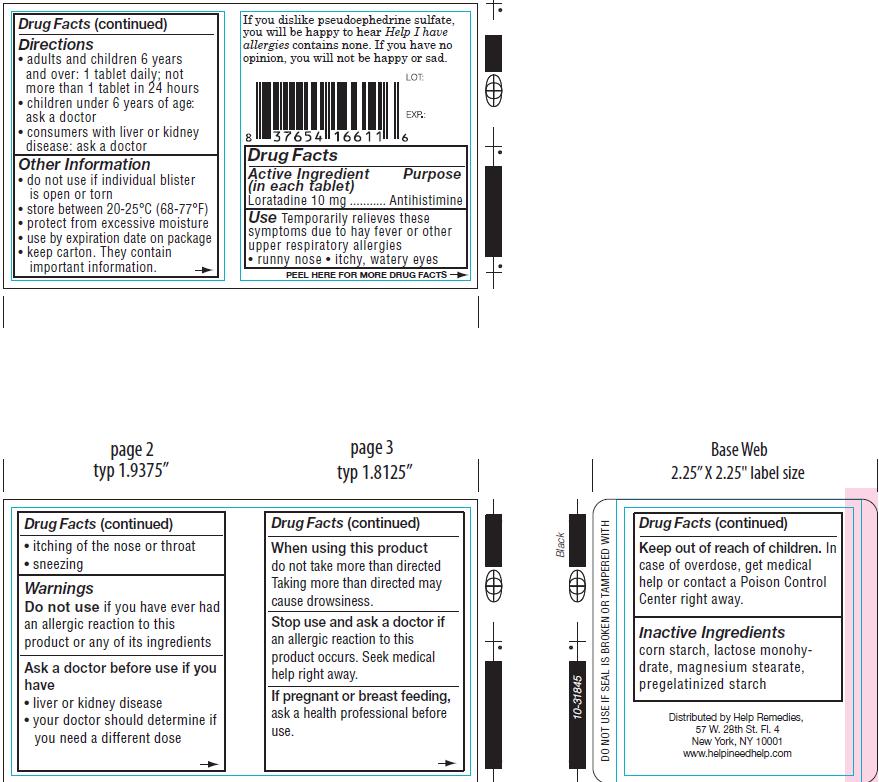
Active Ingredient Purpose
(in each tablet)
Loratadine 10mg..........................................Antihistamine
Use
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies- runny nose
- itchy, watery eyes
- itching of the nose or throat
- sneezing
Warnings
Do not use ifyou have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- liver or kidney disease
- your doctor should determine if you need a different dose
cause drowsiness.
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical
help right away.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control
Center right away.
Directions
- adults and children 6 years and over: 1 tablet daily; not more than 1 tablet in 24 hours
- children under 6 years of age: ask a doctor
- consumers with liver or kidney disease: ask a doctor