Label: TEMPORARY FIBROMYALGIA PAIN AND DISCOMFORT RELIEF- aconitum napellus, atropa belladonna, causticum, magnesium phosphate, dibasic, strychnos nux-vomica seed, toxicodendron pubescens leaf tablet, multilayer
-
Contains inactivated NDC Code(s)
NDC Code(s): 17312-004-12 - Packager: TRP Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated May 31, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Homeopathic Purpose
-
Active Ingredients
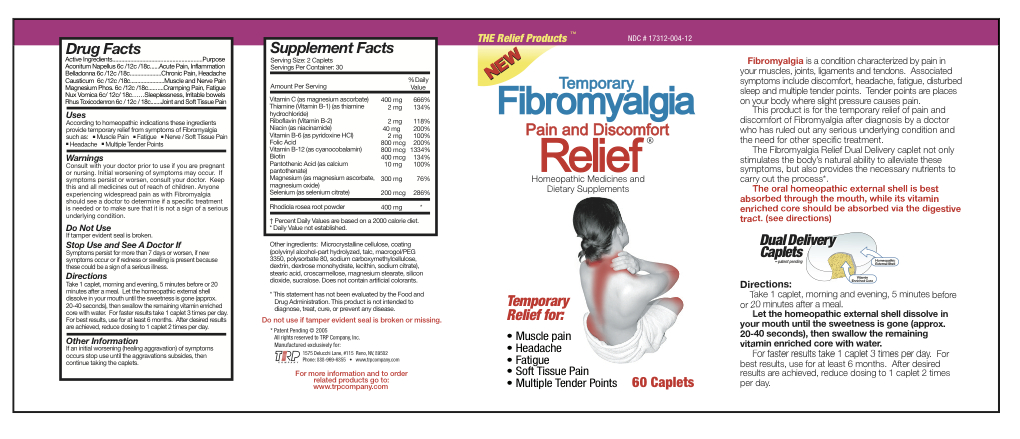
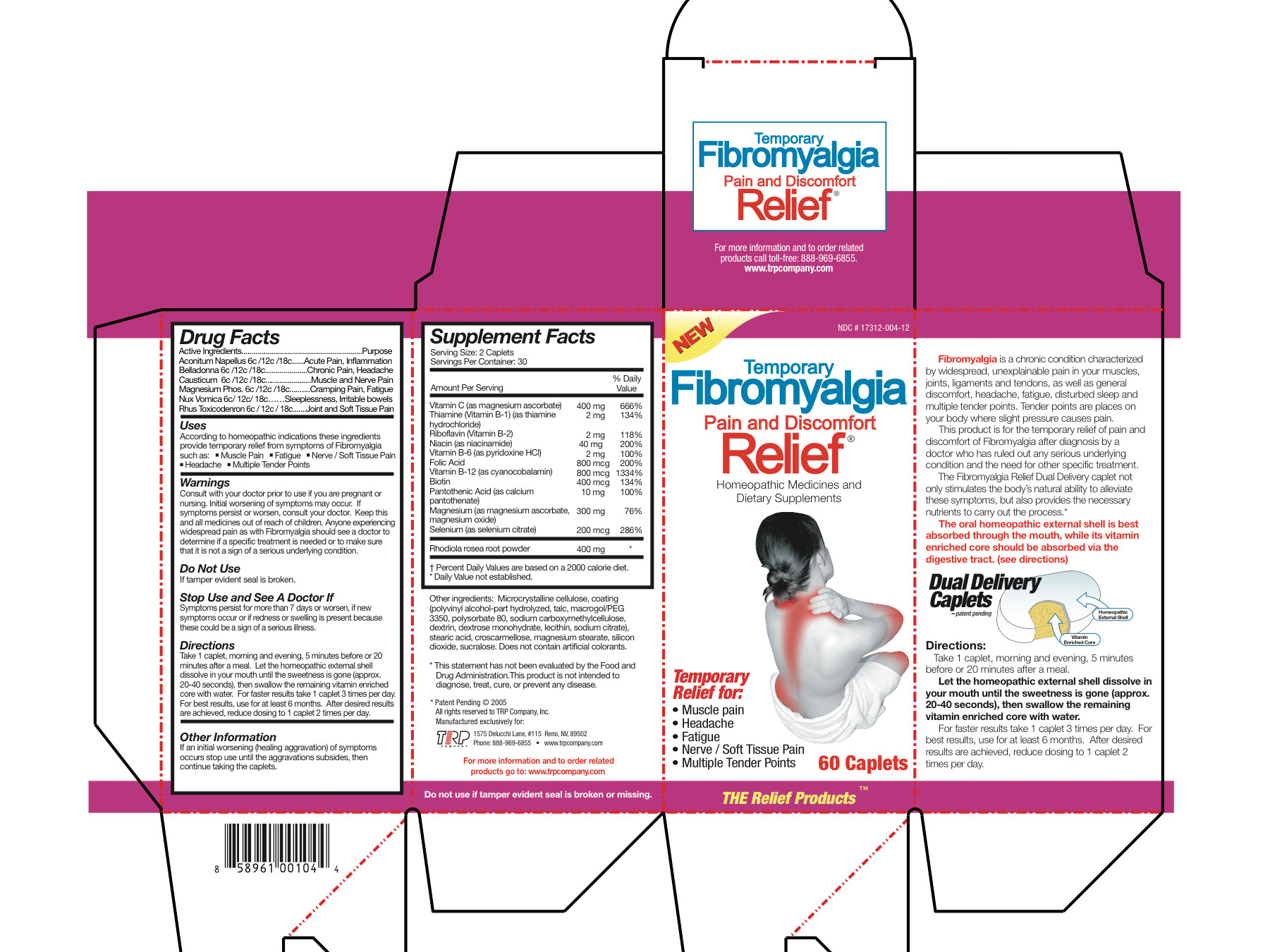
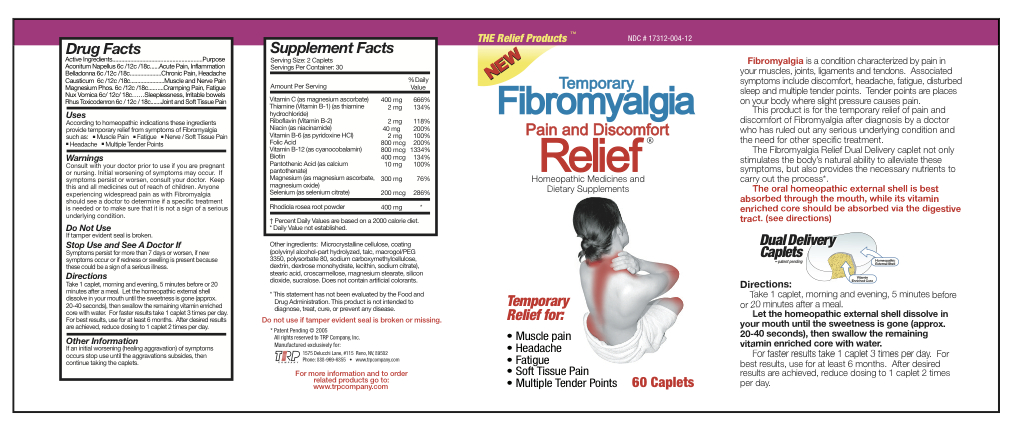
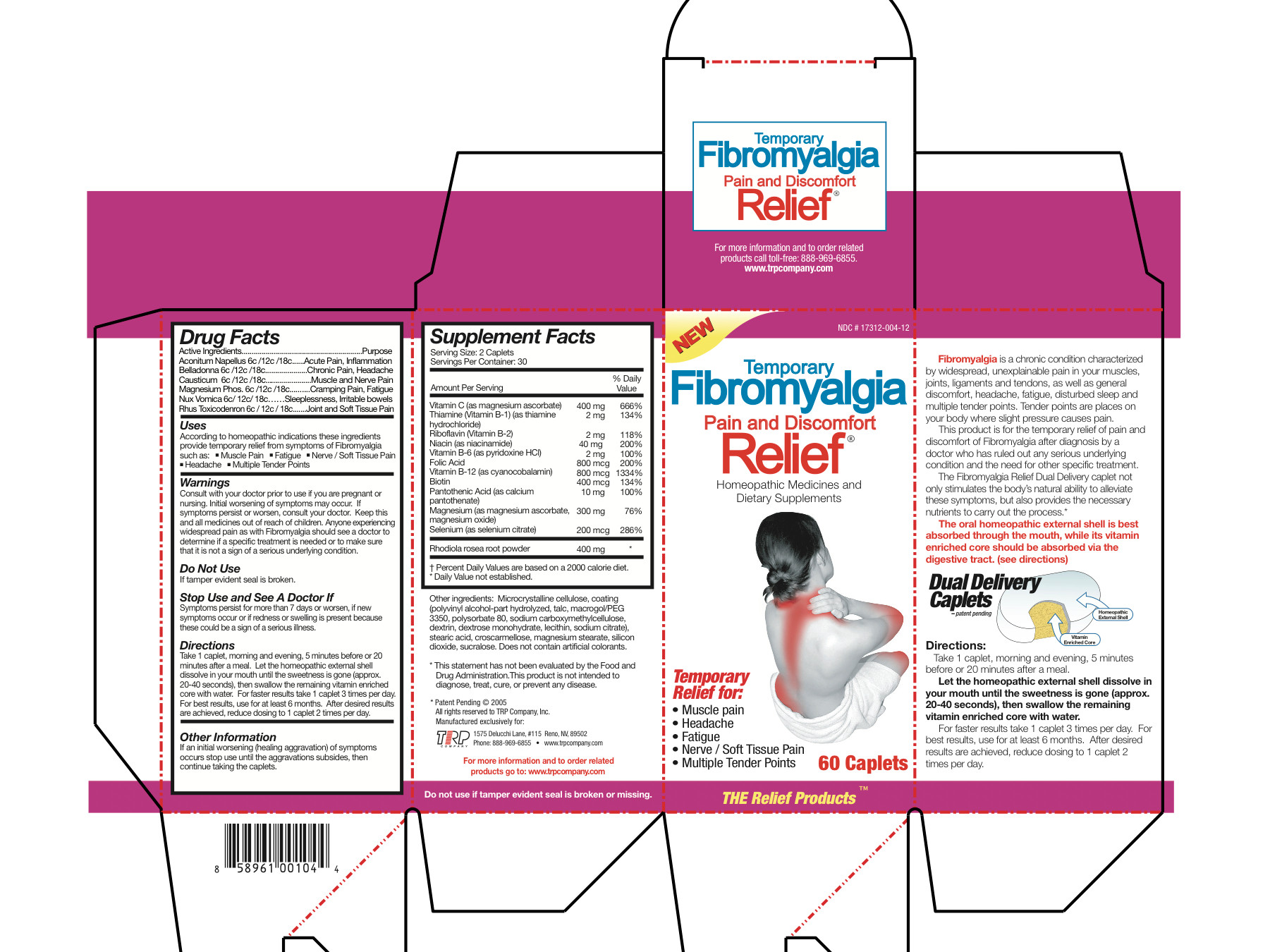
Aconitum Napellus 6c/12c/18c Belladonna 6c/12c/18c Causticum 6c/12c/18c Magnesium Phos 6c/12c/18c Nux Vomica 6c/12c/18c Rhus Toxicodendron 6c/12c/18c Magnesium Ascorbate 200 mg Thiamine Hydrochloride 1 mg Riboflavin 1 mg Niacinamide 20 mg Pyridoxine Hydrochloride 1 mg Folic Acid 400 mcg Cyanocobalamin 400 mcg Biotin 200 mcg Calcium Pantothenate 5 mg Magnesium Ascorbate 75 mg Magnesium Oxide 75 mg Selenium 100 mcg Sedum Roseum Root 200 mg - Inactive Ingredients
- Uses
-
Warnings
- Consult with your doctor prior to use if you are pregnant or nursing.
- Initial worsening of symptoms may occur.
- If symptoms persist or worsen, consult your doctor.
- Anyone experiencing widespread pain as with Fibromyalgia should see a doctor to determine if a specific treatment is needed or to make sure that it is not a sign of a serious underlying condition.
- KEEP OUT OF REACH OF CHILDREN
- Do not use
- Stop Use and See A Doctor If
-
Directions
Take 1 caplet, morning and evening, 5 minutes before or 20 minutes after a meal. Let the homeopathic external shell dissolve in your mouth until the sweetness is gone (approx. 20-40 seconds), then swallow the remaining vitamin enriched core with water. For faster results take 1 caplet 3 times per day. For best results, use for at least 6 months. After desired results are achieved, reduce dosing to 1 caplet 2 times per day.
- Other Information
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TEMPORARY FIBROMYALGIA PAIN AND DISCOMFORT RELIEF
aconitum napellus, atropa belladonna, causticum, magnesium phosphate, dibasic, strychnos nux-vomica seed, toxicodendron pubescens leaf tablet, multilayerProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17312-004 Route of Administration Oral Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Aconitum Napellus (UNII: U0NQ8555JD) (Aconitum Napellus - UNII:U0NQ8555JD) Aconitum Napellus 6 [hp_C] Atropa Belladonna (UNII: WQZ3G9PF0H) (Atropa Belladonna - UNII:WQZ3G9PF0H) Atropa Belladonna 6 [hp_C] Causticum (UNII: DD5FO1WKFU) (Causticum - UNII:DD5FO1WKFU) Causticum 6 [hp_C] Magnesium Phosphate, Dibasic Trihydrate (UNII: HF539G9L3Q) (Magnesium Cation - UNII:T6V3LHY838) Magnesium Phosphate, Dibasic Trihydrate 6 [hp_C] Strychnos Nux-Vomica Seed (UNII: 269XH13919) (Strychnos Nux-Vomica Seed - UNII:269XH13919) Strychnos Nux-Vomica Seed 6 [hp_C] Toxicodendron Pubescens Leaf (UNII: 6IO182RP7A) (Toxicodendron Pubescens Leaf - UNII:6IO182RP7A) Toxicodendron Pubescens Leaf 6 [hp_C] Magnesium Ascorbate (UNII: 0N1G678593) (Magnesium Ascorbate - UNII:0N1G678593) Magnesium Ascorbate 200 mg Thiamine Hydrochloride (UNII: M572600E5P) (Thiamine Ion - UNII:4ABT0J945J) Thiamine Hydrochloride 1 mg Riboflavin (UNII: TLM2976OFR) (Riboflavin - UNII:TLM2976OFR) Riboflavin 1 mg Niacinamide (UNII: 25X51I8RD4) (Niacinamide - UNII:25X51I8RD4) Niacinamide 20 mg Pyridoxine Hydrochloride (UNII: 68Y4CF58BV) (Pyridoxine - UNII:KV2JZ1BI6Z) Pyridoxine Hydrochloride 1 mg Folic Acid (UNII: 935E97BOY8) (Folic Acid - UNII:935E97BOY8) Folic Acid 400 ug Cyanocobalamin (UNII: P6YC3EG204) (Cyanocobalamin - UNII:P6YC3EG204) Cyanocobalamin 400 ug Biotin (UNII: 6SO6U10H04) (Biotin - UNII:6SO6U10H04) Biotin 200 ug Calcium Pantothenate (UNII: 568ET80C3D) (Calcium Pantothenate - UNII:568ET80C3D) Calcium Pantothenate 5 mg Magnesium Ascorbate (UNII: 0N1G678593) (Magnesium Ascorbate - UNII:0N1G678593) Magnesium Ascorbate 75 mg Magnesium Oxide (UNII: 3A3U0GI71G) (Magnesium Oxide - UNII:3A3U0GI71G) Magnesium Oxide 75 mg Selenium (UNII: H6241UJ22B) (Selenium - UNII:H6241UJ22B) Selenium 100 ug Sedum Roseum Root (UNII: 3S5ITS5ULN) (Sedum Roseum Root - UNII:3S5ITS5ULN) Sedum Roseum Root 200 mg Inactive Ingredients Ingredient Name Strength Cellulose, Microcrystalline (UNII: OP1R32D61U) Polyvinyl Alcohol (UNII: 532B59J990) Talc (UNII: 7SEV7J4R1U) Polyethylene Glycol 3350 (UNII: G2M7P15E5P) Polysorbate 80 (UNII: 6OZP39ZG8H) Carboxymethylcellulose Sodium (UNII: K679OBS311) Icodextrin (UNII: 2NX48Z0A9G) Dextrose Monohydrate (UNII: LX22YL083G) Egg Phospholipids (UNII: 1Z74184RGV) Sodium Citrate (UNII: 1Q73Q2JULR) Stearic Acid (UNII: 4ELV7Z65AP) Croscarmellose (UNII: 029TFK992N) Magnesium Stearate (UNII: 70097M6I30) Silicon Dioxide (UNII: ETJ7Z6XBU4) Sucralose (UNII: 96K6UQ3ZD4) Product Characteristics Color white Score no score Shape OVAL (Tablet) Size 9mm Flavor Imprint Code None Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17312-004-12 1 in 1 Package 1 60 in 1 Bottle Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/01/2010 Labeler - TRP Company (105185719)