Homeopathic Purpose
| Aconitum Napellus | 6c/12c/18c | Acute Pain, Inflammation |
| Belladonna | 6c/12c/18c | Chronic Pain, Headache |
| Causticum | 6c/12c/18c | Muscle and Nerve Pain |
| Magnesium Phos | 6c/12c/18c | Cramping Pain, Fatigue |
| Nux Vomica | 6c/12c/18c | Sleeplessness, Irritable bowels |
| Rhus Toxicodendron | 6c/12c/18c | Joint and Soft Tissue Pain |
Active Ingredients
| Aconitum Napellus | 6c/12c/18c |
| Belladonna | 6c/12c/18c |
| Causticum | 6c/12c/18c |
| Magnesium Phos | 6c/12c/18c |
| Nux Vomica | 6c/12c/18c |
| Rhus Toxicodendron | 6c/12c/18c |
| Magnesium Ascorbate | 200 mg |
| Thiamine Hydrochloride | 1 mg |
| Riboflavin | 1 mg |
| Niacinamide | 20 mg |
| Pyridoxine Hydrochloride | 1 mg |
| Folic Acid | 400 mcg |
| Cyanocobalamin | 400 mcg |
| Biotin | 200 mcg |
| Calcium Pantothenate | 5 mg |
| Magnesium Ascorbate | 75 mg |
| Magnesium Oxide | 75 mg |
| Selenium | 100 mcg |
| Sedum Roseum Root | 200 mg |
Inactive Ingredients
| Microcrystalline cellulose |
| Polyvinyl Alcohol |
| Talc |
| Macrogol/PEG 3350 |
| Polysorbate 80 |
| Sodium Carboxymethylcellulose |
| Dextrin |
| Dextrose Monohydrate |
| Lecithin |
| Sodium Citrate |
| Stearic Acid |
| Croscarmellose |
| Magnesium Stearate |
| Silicon Dioxide |
| Sucralose |
Uses
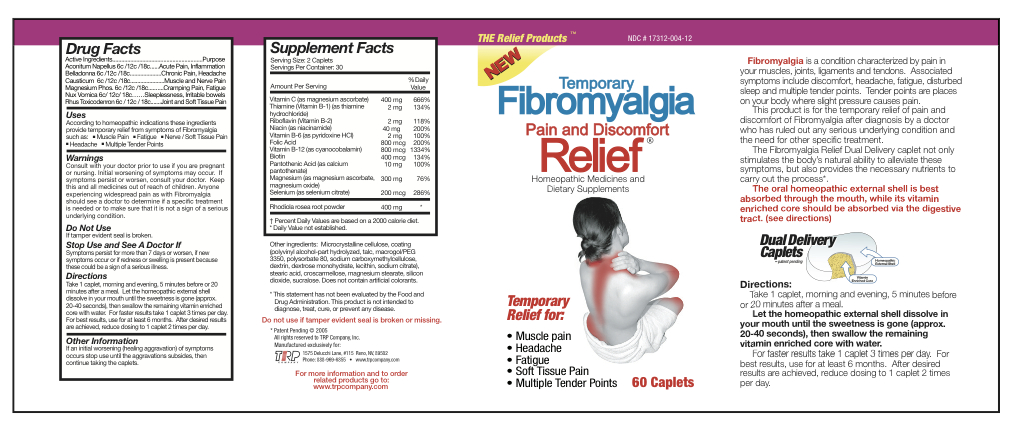
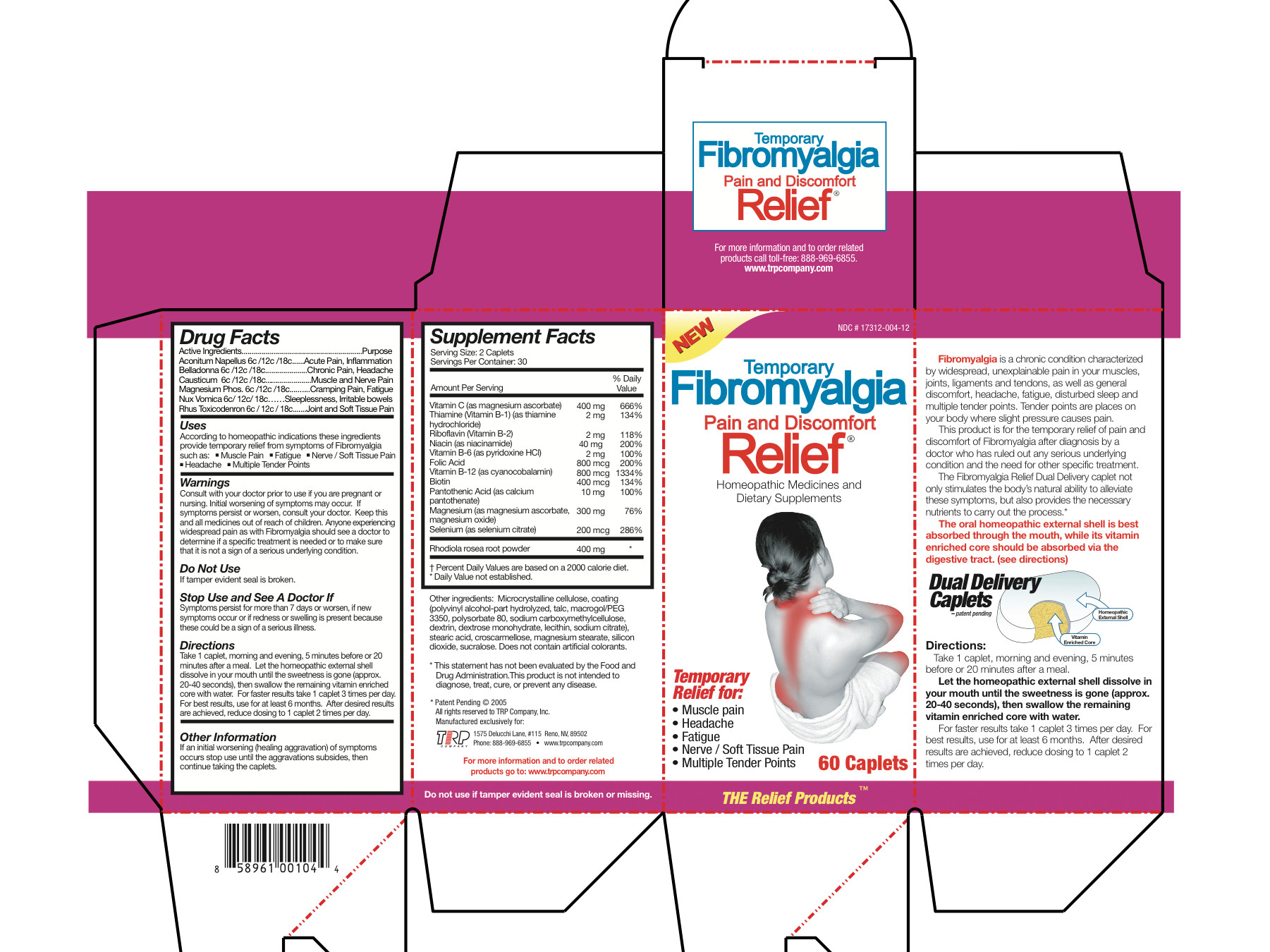
According to homeopathic indications these ingredients provide temporary relief from symptoms of Fibromyalgia such as:
- Muscle Pain
- Fatigue
- Nerve/Soft Tissue Pain
- Headache
- Multiple Tender Points
Warnings
- Consult with your doctor prior to use if you are pregnant or nursing.
- Initial worsening of symptoms may occur.
- If symptoms persist or worsen, consult your doctor.
- Anyone experiencing widespread pain as with Fibromyalgia should see a doctor to determine if a specific treatment is needed or to make sure that it is not a sign of a serious underlying condition.
Stop Use and See A Doctor If
If Symptoms persist for more than 7 days or worsen or if new symptoms occur or if redness or swelling is present because these could be a sign of a serious illness.
Directions
Take 1 caplet, morning and evening, 5 minutes before or 20 minutes after a meal.
Let the homeopathic external shell dissolve in your mouth until the sweetness is
gone (approx. 20-40 seconds), then swallow the remaining vitamin enriched core
with water. For faster results take 1 caplet 3 times per day. For best results,
use for at least 6 months. After desired results are achieved, reduce dosing to 1
caplet 2 times per day.