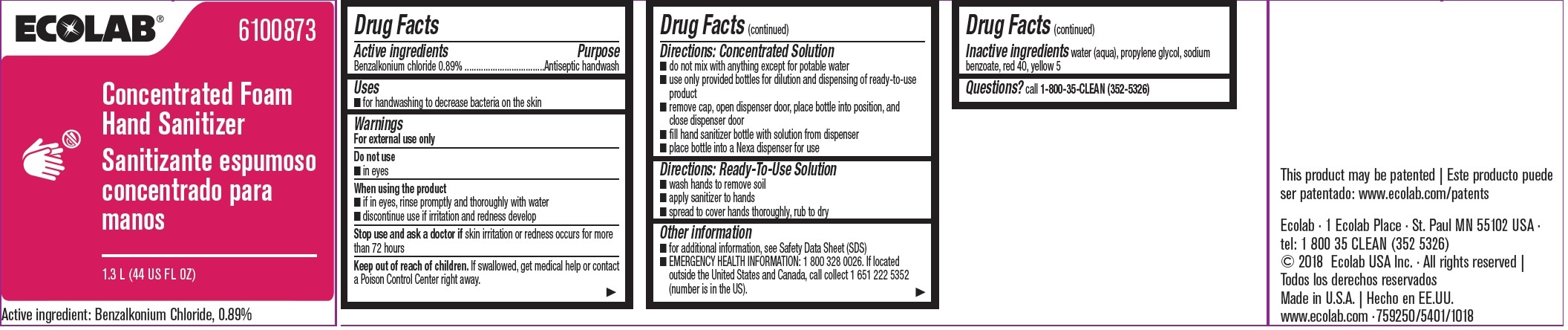
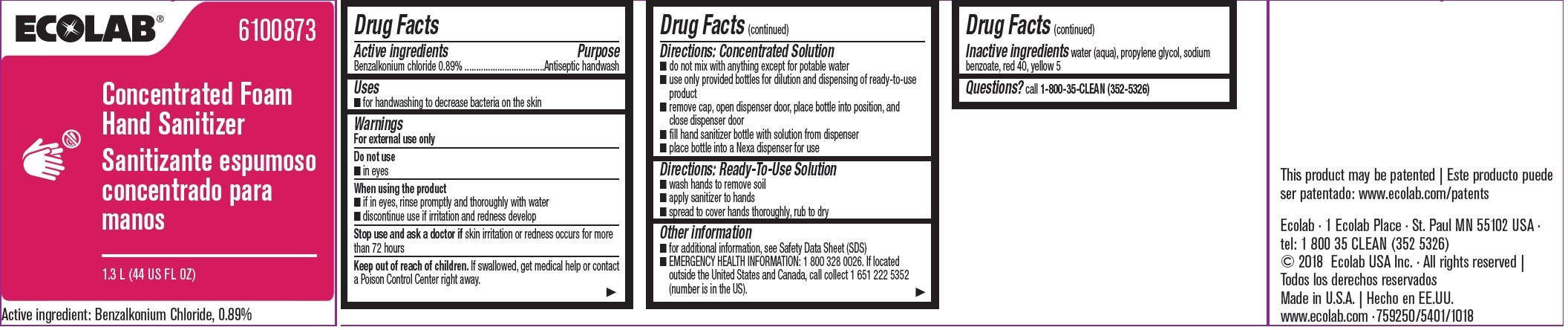
Label: ECOLAB INC.- benzalkonium chloride solution
- NDC Code(s): 47593-512-61, 47593-512-62, 47593-512-94
- Packager: Ecolab Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated May 3, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions: Concentrated Solution
- do not mix with anything except for potable water
- use only provided bottles for dilution and dispensing of ready-to-use product
- remove cap, open dispenser door, place bottle into position, and close dispenser door
- fill hand sanitizer bottle with solution form dispenser
- place bottle into a Nexa dispenser for use
- Directions: Ready-To-Use Solution
- Other information
- INACTIVE INGREDIENT
- QUESTIONS
- Principal display panel and representative label
-
INGREDIENTS AND APPEARANCE
ECOLAB INC.
benzalkonium chloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47593-512 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 8.9 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM BENZOATE (UNII: OJ245FE5EU) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C RED NO. 40 (UNII: WZB9127XOA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47593-512-61 2000 mL in 1 POUCH; Type 0: Not a Combination Product 03/02/2015 2 NDC:47593-512-62 1300 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/02/2015 3 NDC:47593-512-94 1900 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/02/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 03/02/2015 Labeler - Ecolab Inc. (006154611)