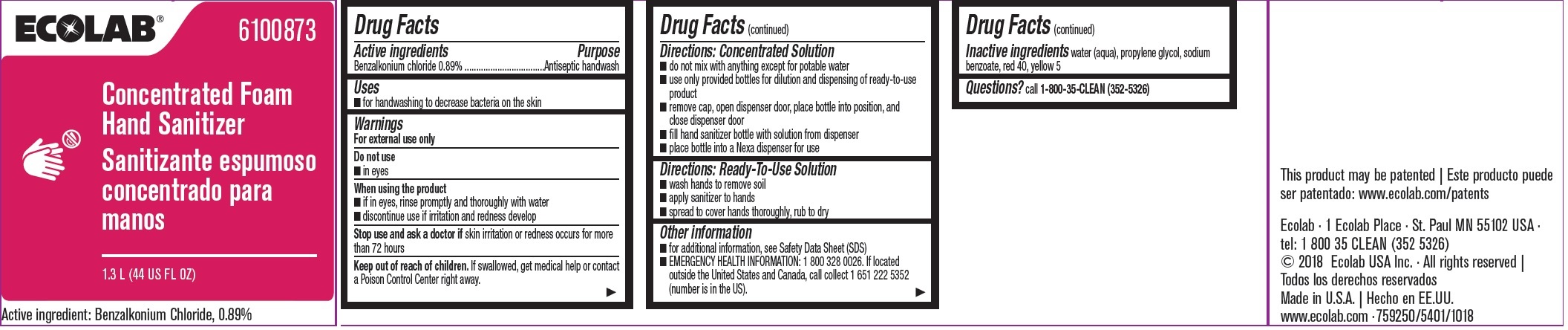
Warnings
For external use only
Directions: Concentrated Solution
- do not mix with anything except for potable water
- use only provided bottles for dilution and dispensing of ready-to-use product
- remove cap, open dispenser door, place bottle into position, and close dispenser door
- fill hand sanitizer bottle with solution form dispenser
- place bottle into a Nexa dispenser for use
Directions: Ready-To-Use Solution
- wash hands to remove soil
- apply sanitizer to hands
- spread to cover hands thoroughly, rub to dry
Other information
- for additional information, see Safety Data Sheet (SDS)
- EMERGENCY HEALTH INFORMATION: 1 800 328 0026. If located outside the United States and Canada, call collect 1 651 222 5352 (number is in the US).
Principal display panel and representative label
ECOLAB® 6100873
Concentrated Foam1.3 L (44 US FL OZ)
Active ingredient: Benzalkonium chloride, 0.89%
Ecolab - 1 Ecolab Place - St. Paul MN 55102 USA
tel: 1 800 35 CLEAN (352 5326)
© 2018 Ecolab USA Inc • All rights reserved
Made in U.S.A.
www.ecolab.com • 759250/5401/1018