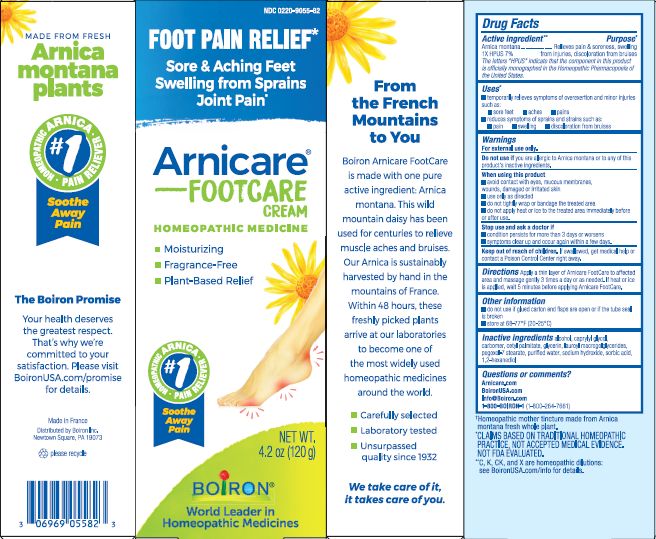
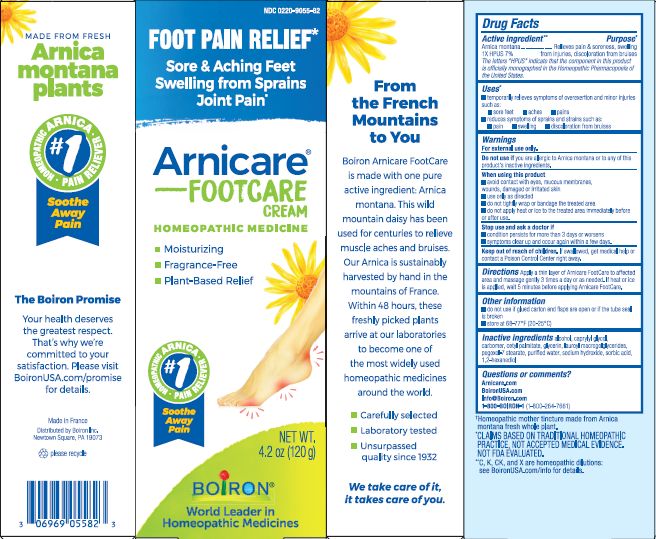
Label: ARNICARE FOOT CARE- arnica montana cream
- NDC Code(s): 0220-9055-82
- Packager: Boiron
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated May 23, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
SPL UNCLASSIFIED SECTION
do not use if glued carton end flaps are open or if the tube seal is broken,
store at 68-77° F (20-25° C)
4.2 oz (120g)
Moisturizing
Fragrance-Free
Plant-Based Relief
Sore & Aching Feet Swelling from Sprains Joint Pain*
Foot Pain Relief*
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
*C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details. - INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ARNICARE FOOT CARE
arnica montana creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0220-9055 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 1 [hp_X] in 1 [hp_X] Inactive Ingredients Ingredient Name Strength HYDROGENATED PALM/PALM KERNEL OIL PEG-6 ESTERS (UNII: 8EPU9MJ01K) WATER (UNII: 059QF0KO0R) CARBOMER 934 (UNII: Z135WT9208) ALCOHOL (UNII: 3K9958V90M) SORBIC ACID (UNII: X045WJ989B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GLYCERIN (UNII: PDC6A3C0OX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) SODIUM HYDROXIDE (UNII: 55X04QC32I) PEGOXOL 7 STEARATE (UNII: 3EW5AXE5X5) CETYL PALMITATE (UNII: 5ZA2S6B08X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0220-9055-82 1 in 1 PACKAGE 06/01/2018 1 1 [hp_X] in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/01/2018 Labeler - Boiron (282560473) Registrant - Boiron Inc. (014892269) Establishment Name Address ID/FEI Business Operations Boiron 282560473 manufacture(0220-9055)