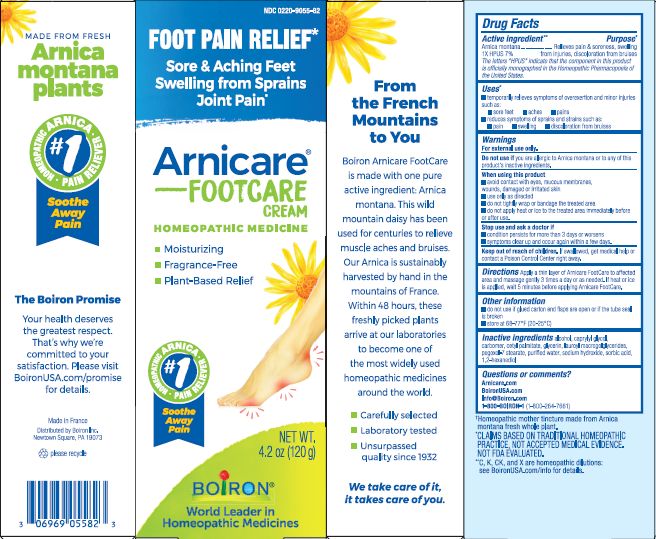
Active Ingredient **
Arnica montana 1X HPUS 7%
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
Uses*
temporarily relieves symptoms of overexertion and minor injuries such as:
- sore feet
- aches
- pains
- reduces symptoms of sprains and strains such as:
- pain
- swelling
- discoloration from bruises
When using this product
- avoid contact with eyes, mucous membranes, wounds, damaged or irritated skin
- use only as directed
- do not tightly wrap or bandage the treated area
- do not apply heat or ice to the treated area immediately before or after use.
Stop use and ask a doctor if
- condition persists for more than 3 days or worsen
- symptoms clear up and occur again within a few days.
Keep out of reach of children. If swallowed, get medical help or contact Poions Control Center right away.
Directions
Apply a thin layer of Arnicare FootCare to affected area and massage gently 3 times a day or as needed. If heat or ice is applied, wait 5 minutes before applying Arnicare FootCare.
do not use if glued carton end flaps are open or if the tube seal is broken,
store at 68-77° F (20-25° C)
4.2 oz (120g)
Moisturizing
Fragrance-Free
Plant-Based Relief
Sore & Aching Feet Swelling from Sprains Joint Pain*
Foot Pain Relief*
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
*C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.
alcohol, caprylyl glycol, carbomer, cetyl palmitate, glycerin, lauroyl macrogolglycerides, pexoxol-7 stearate, purified water, sodium hydroxide, sorbic acid, 1,2-hexanediol