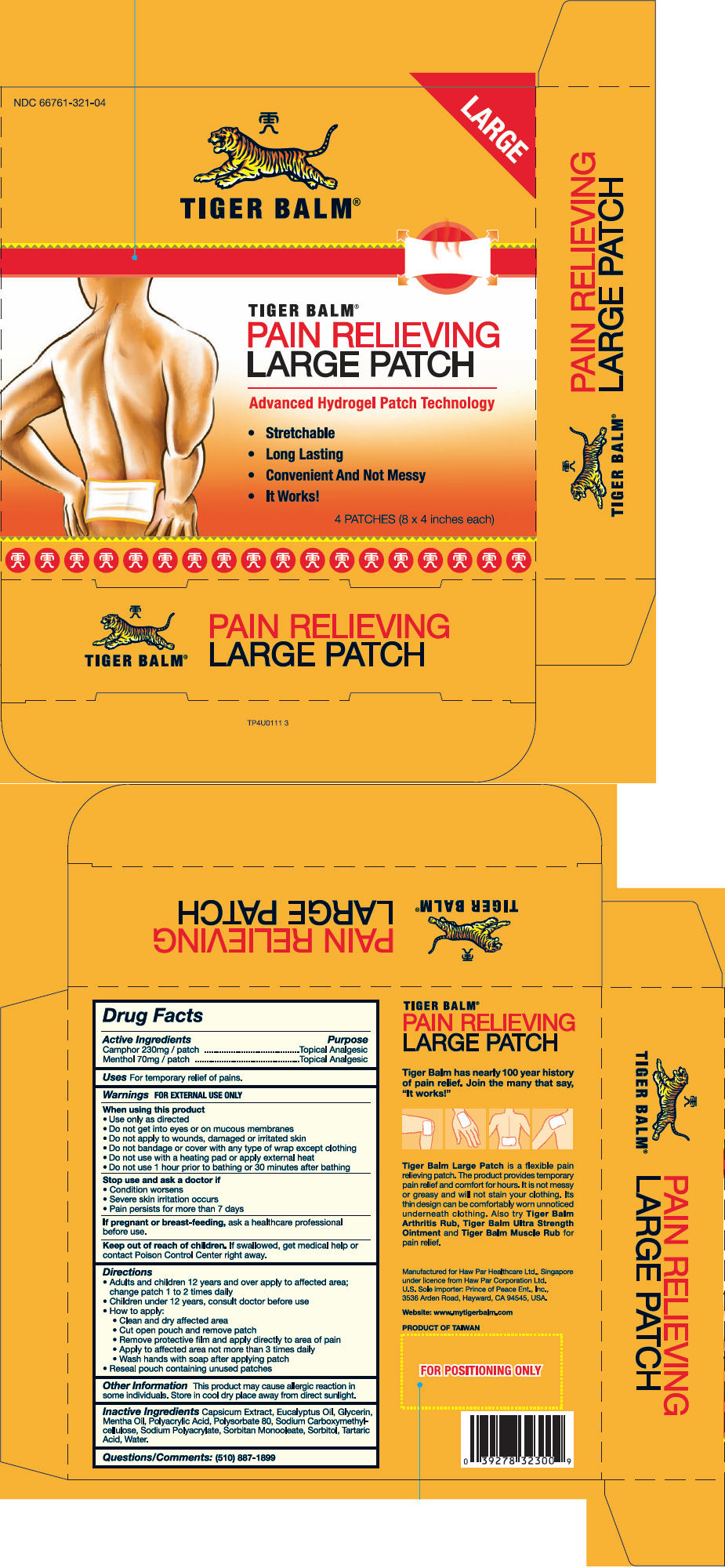
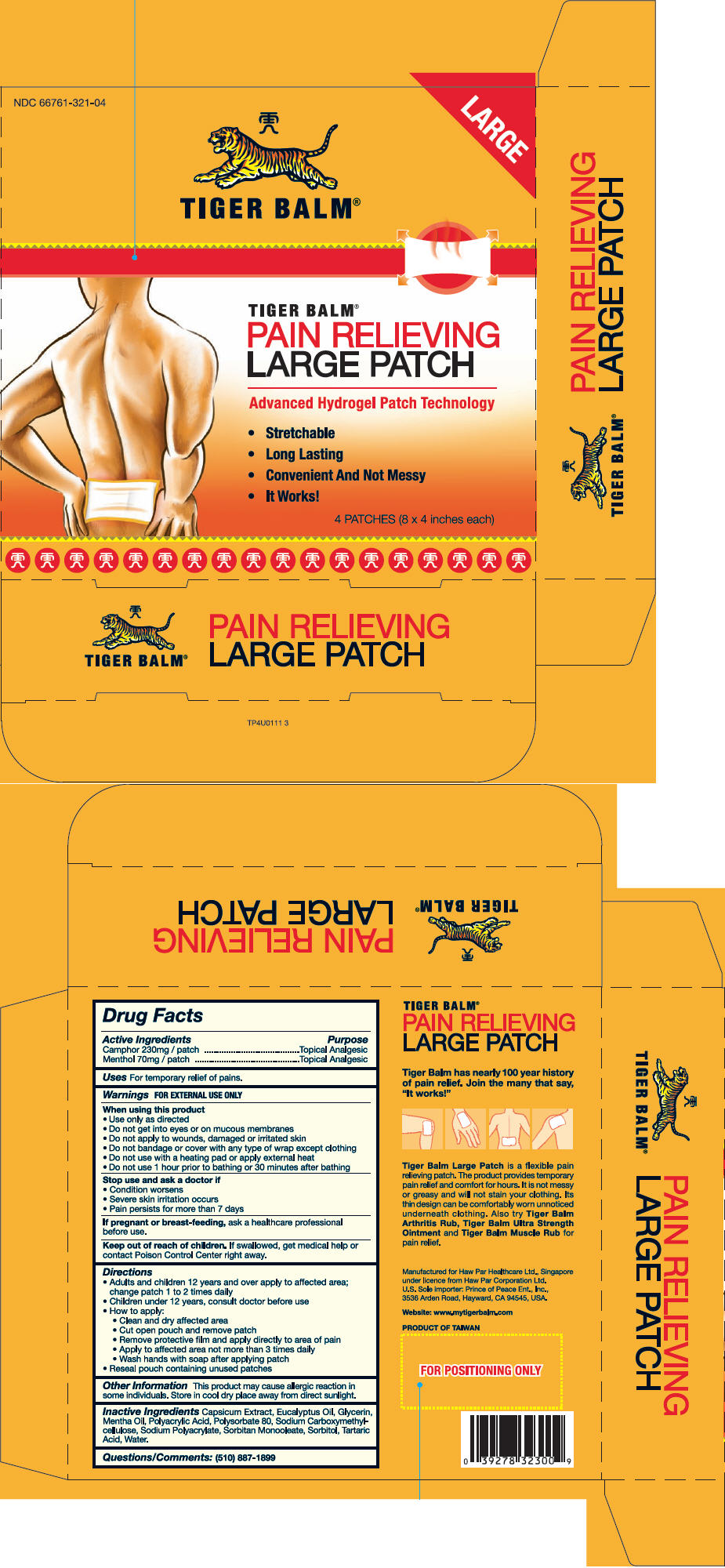
Label: TIGER BALM (camphor- synthetic and menthol patch
- NDC Code(s): 66761-321-04
- Packager: Haw Par Healthcare Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
FOR EXTERNAL USE ONLY
When using this product
- Use only as directed
- Do not get into eyes or on mucous membranes
- Do not apply to wounds, damaged or irritated skin
- Do not bandage or cover with any type of wrap except clothing
- Do not use with a heating pad or apply external heat
- Do not use 1 hour prior to bathing or 30 minutes after bathing
-
Directions
- Adults and children 12 years and over apply to affected area; change patch 1 to 2 times daily
- Children under 12 years, consult doctor before use
- How to apply:
- Clean and dry affected area
- Cut open pouch and remove patch
- Remove protective film and apply directly to area of pain
- Apply to affected area not more than 3 times daily
- Wash hands with soap after applying patch
- Reseal pouch containing unused patches
- Other Information
- Inactive Ingredients
- Questions/Comments
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 4 Patch Carton
-
INGREDIENTS AND APPEARANCE
TIGER BALM
camphor (synthetic) and menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66761-321 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Camphor (Synthetic) (UNII: 5TJD82A1ET) (Camphor (Synthetic) - UNII:5TJD82A1ET) Camphor (Synthetic) 230 mg MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 70 mg Inactive Ingredients Ingredient Name Strength Eucalyptus Oil (UNII: 2R04ONI662) Glycerin (UNII: PDC6A3C0OX) Polysorbate 80 (UNII: 6OZP39ZG8H) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) Sorbitan Monooleate (UNII: 06XEA2VD56) Sorbitol (UNII: 506T60A25R) Tartaric Acid (UNII: W4888I119H) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66761-321-04 1 in 1 BOX 01/15/2007 1 4 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M017 01/15/2007 Labeler - Haw Par Healthcare Ltd. (659207039) Establishment Name Address ID/FEI Business Operations Teh Seng Pharmaceutical Mfg. Co., Ltd. 656347721 MANUFACTURE(66761-321)