Label: BENZOCAINE swab
- NDC Code(s): 67777-246-01, 67777-246-02, 67777-246-03, 67777-246-04
- Packager: Dynarex Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Use(s)
-
Warnings
For External Use Only
• Flammable, keep away from fire or flame
• Avoid contact with eyes; if this occures, rinse thoroughly with water
• Do not use with electrocautery procedures
- Directions
- Other Information
- Inactive Ingredients
- Questions?
- Label
- Label 1408UB-10
-
INGREDIENTS AND APPEARANCE
BENZOCAINE
benzocaine swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67777-246 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 60 mg in 100 mL BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 6 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape RECTANGLE Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67777-246-02 3000 in 1 CASE 07/20/2011 1 NDC:67777-246-01 2 mL in 1 PACKET; Type 0: Not a Combination Product 2 NDC:67777-246-04 480 in 1 CASE 07/20/2011 2 NDC:67777-246-03 10 in 1 BOX 2 2 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 07/20/2011 Labeler - Dynarex Corporation (008124539)

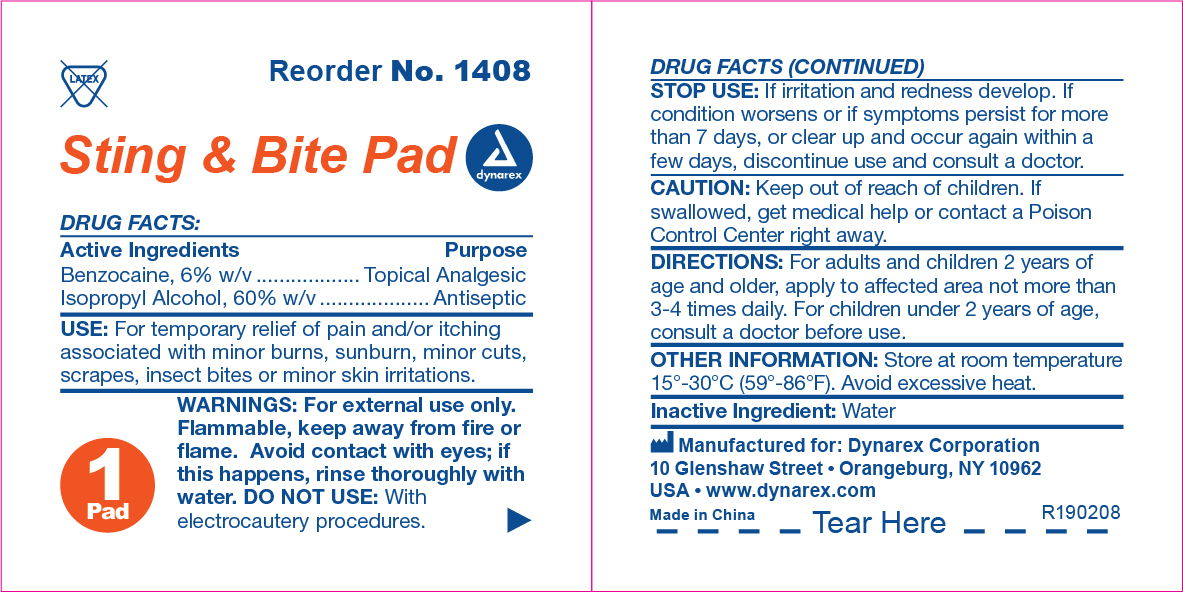
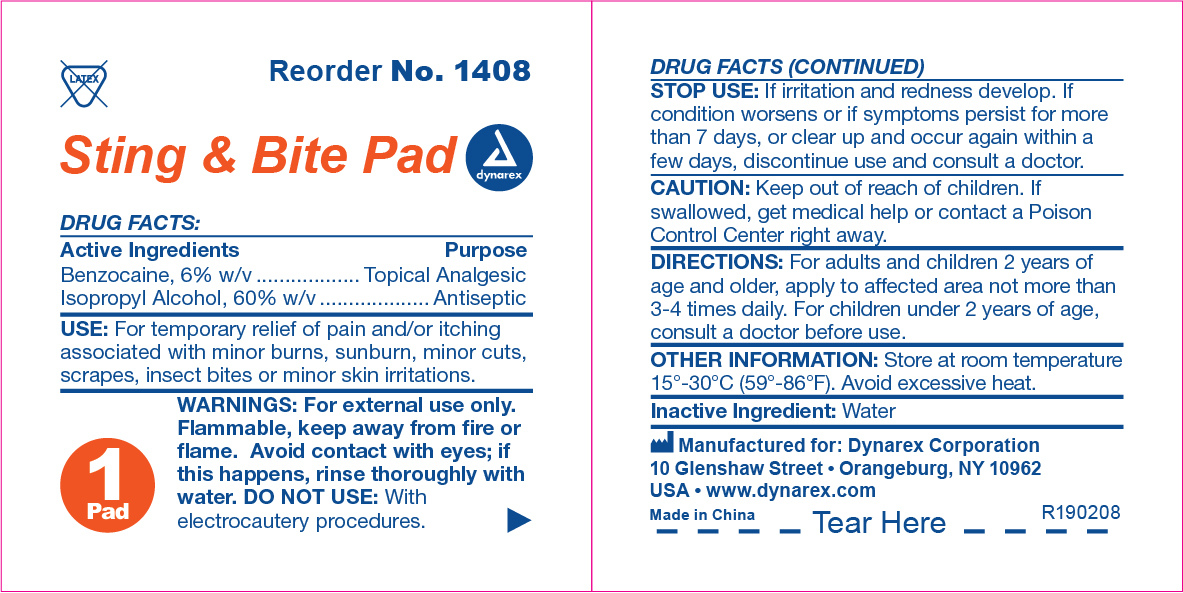 Sting & Bite Pad Label
Sting & Bite Pad Label
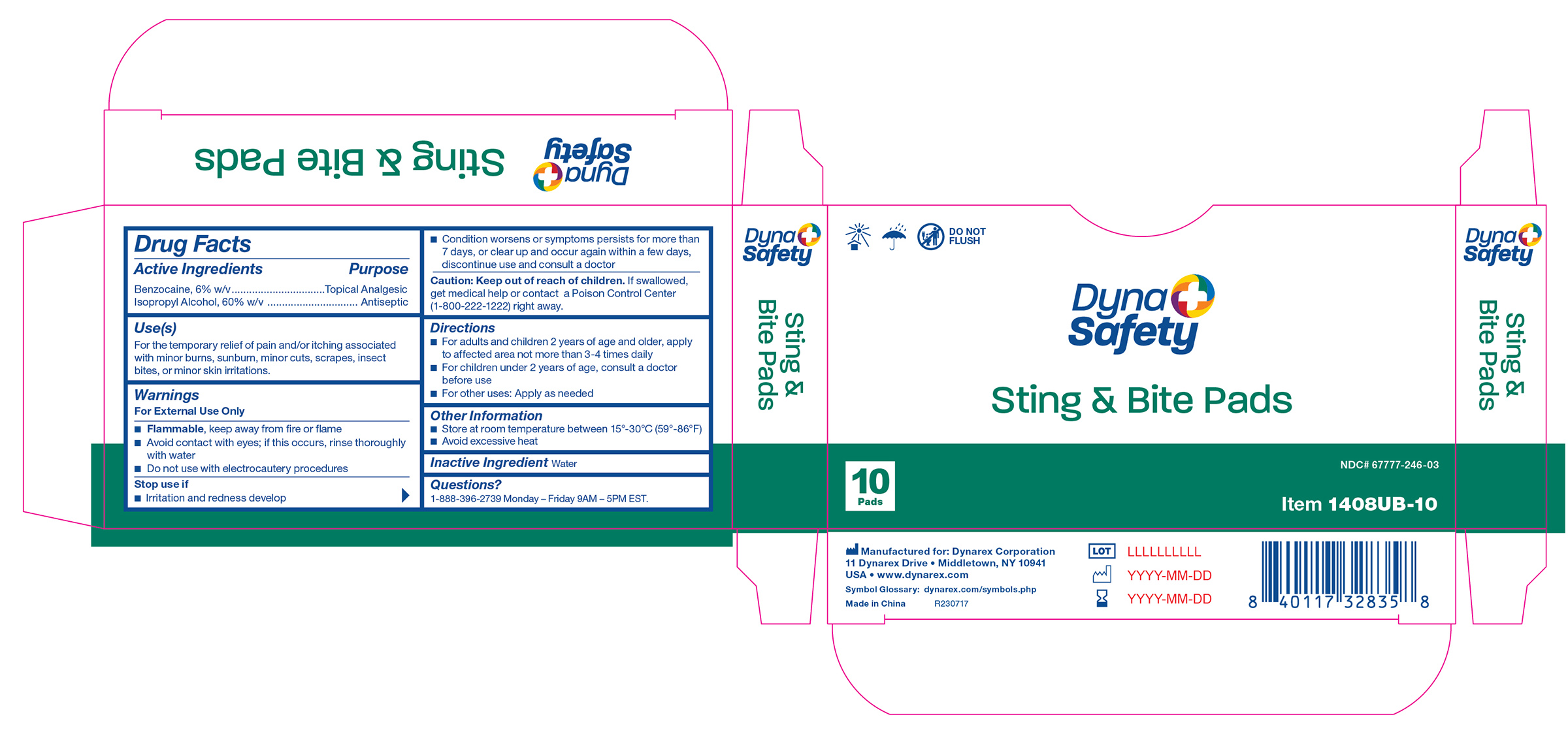
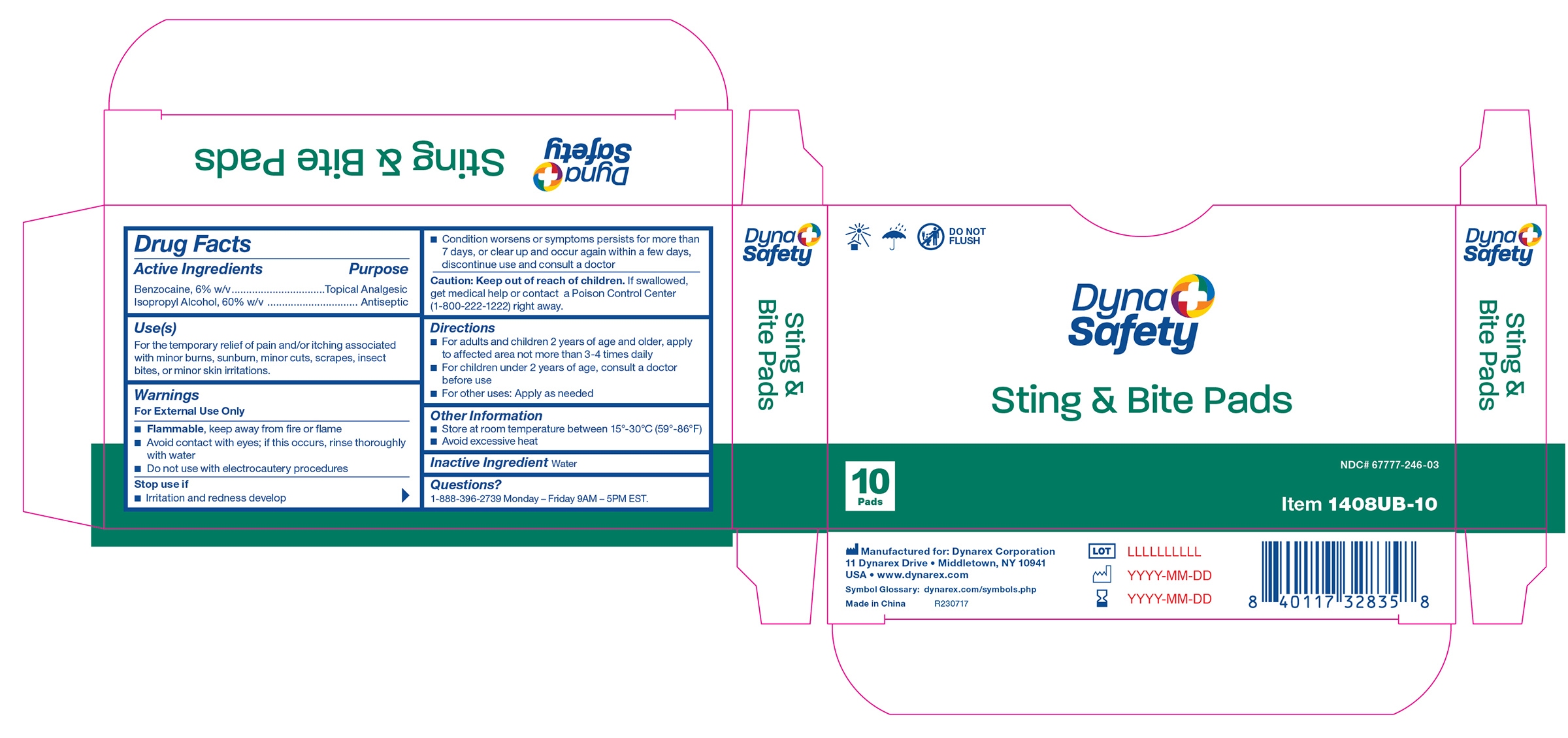 1408UB-10
1408UB-10