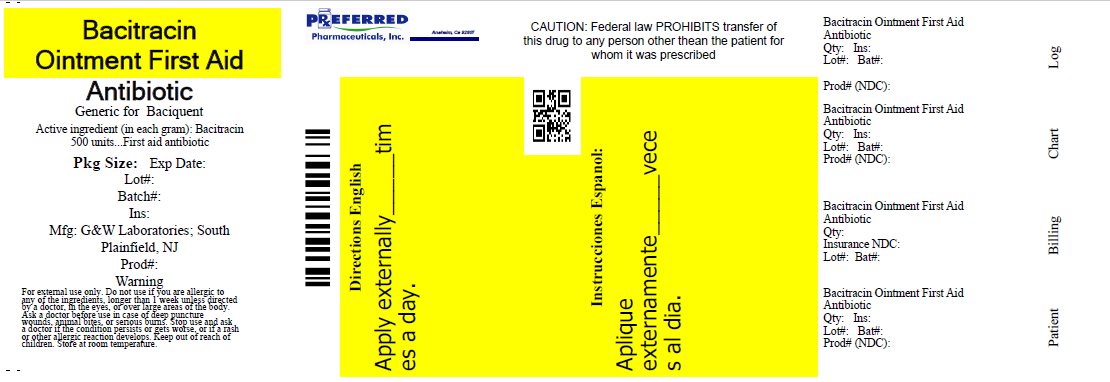
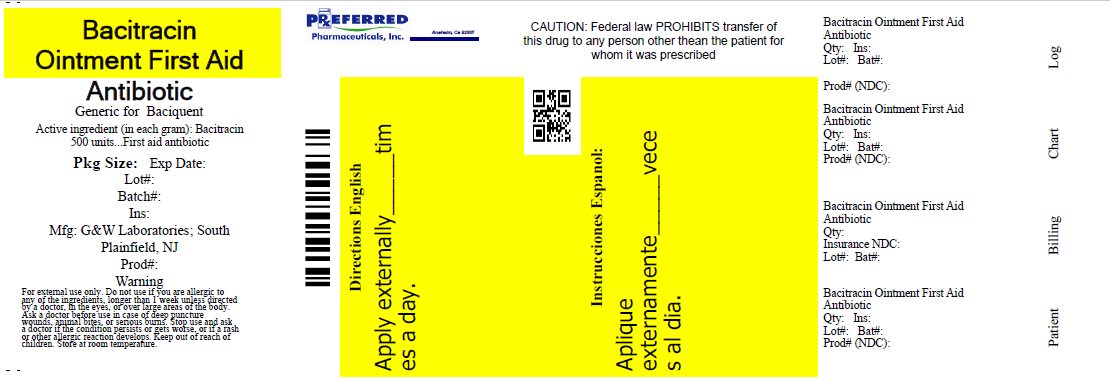
Label: BACITRACIN ointment
- NDC Code(s): 68788-9794-1, 68788-9794-2
- Packager: Preferred Pharmaceuticals, Inc
- This is a repackaged label.
- Source NDC Code(s): 0713-0280
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated February 26, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USES
-
WARNINGS
For external use only
Do not use
• if you are allergic to any of the ingredients
• in the eyes
• over large areas of the body
• longer than 1 week unless directed by a doctor
Ask a doctor before use in case of deep or puncture wounds, animal bites, or serious burns
Stop use and ask a doctor if
• the condition persists or gets worse
• a rash or other allergic reaction develops - KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENT
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BACITRACIN
bacitracin ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68788-9794(NDC:0713-0280) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Bacitracin (UNII: 58H6RWO52I) (Bacitracin - UNII:58H6RWO52I) Bacitracin 500 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength Light Mineral Oil (UNII: N6K5787QVP) Petrolatum (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68788-9794-1 14 g in 1 TUBE; Type 0: Not a Combination Product 04/30/2012 06/12/2019 2 NDC:68788-9794-2 28 g in 1 TUBE; Type 0: Not a Combination Product 04/30/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 333B 04/30/2012 Labeler - Preferred Pharmaceuticals, Inc (791119022) Registrant - Preferred Pharmaceuticals, Inc (791119022) Establishment Name Address ID/FEI Business Operations Preferred Pharmaceuticals, Inc 791119022 RELABEL(68788-9794)