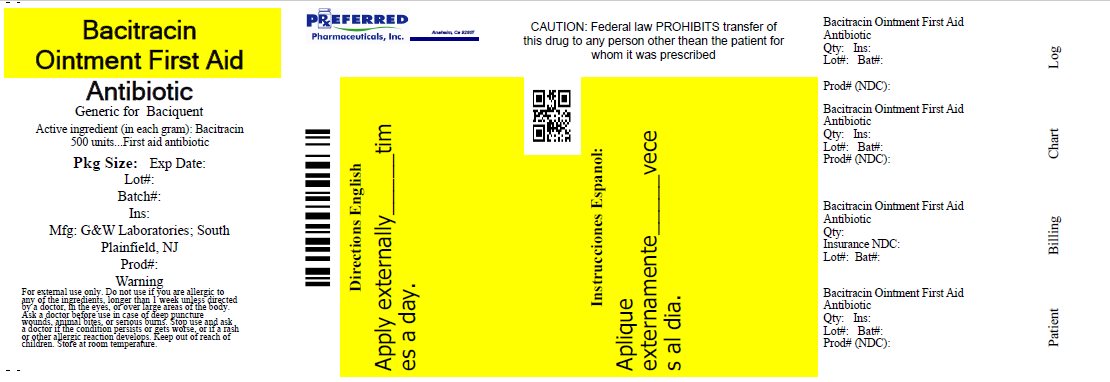
ACTIVE INGREDIENT
Bacitracin 500 units
PURPOSE
First aid antibiotic
USES
first aid to help prevent infection in minor cuts, scrapes and burns
WARNINGS
For external use only
Do not use
• if you are allergic to any of the ingredients
• in the eyes
• over large areas of the body
• longer than 1 week unless directed by a doctor
Ask a doctor before use in case of deep or puncture wounds, animal bites, or serious burns
Stop use and ask a doctor if
• the condition persists or gets worse
• a rash or other allergic reaction develops
KEEP OUT OF REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
• clean the affected area
• apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
• may be covered with a sterile bandage
OTHER INFORMATION
store at room temperature
INACTIVE INGREDIENT
light mineral oil, white petrolatum
HOW SUPPLIED
28gm tube – 68788-9794-2
Relabeled By: Preferred Pharmaceuticals, Inc
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL