Label: CIPRO HC- ciprofloxacin hydrochloride suspension
-
Contains inactivated NDC Code(s)
NDC Code(s): 16590-053-10 - Packager: Stat Rx USA
- This is a repackaged label.
- Source NDC Code(s): 0065-8531
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated October 27, 2009
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
DESCRIPTION
CIPRO® HC OTIC (ciprofloxacin hydrochloride and hydrocortisone otic suspension) contains the synthetic broad spectrum antibacterial agent, ciprofloxacin hydrochloride, combined with the anti-inflammatory corticosteroid, hydrocortisone, in a preserved, nonsterile suspension for otic use. Each mL of CIPRO® HC OTIC contains ciprofloxacin hydrochloride (equivalent to 2 mg ciprofloxacin), 10 mg hydrocortisone, and 9 mg benzyl alcohol as a preservative. The inactive ingredients are polyvinyl alcohol, sodium chloride, sodium acetate, glacial acetic acid, phospholipon 90H (modified lecithin), polysorbate, and purified water. Sodium hydroxide or hydrochloric acid may be added for adjustment of pH.
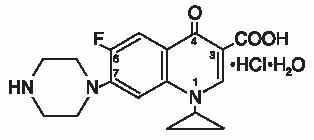
Ciprofloxacin, a fluoroquinolone, is available as the monohydrochloride monohydrate salt of 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid. Its empirical formula is C17H18FN3O3•HCI•H2O and its chemical structure is as follows:
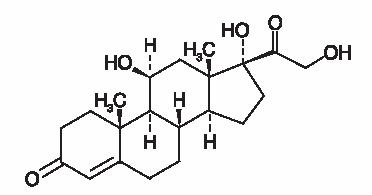
Hydrocortisone, pregn-4-ene-3, 20-dione, 11, 17, 21-trihydroxy-(11β)-, is an anti-inflammatory corticosteroid. Its empirical formula is C21H30O5 and its chemical structure is:
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
The plasma concentrations of ciprofloxacin were not measured following three drops of otic suspension administration because the systemic exposure to ciprofloxacin is expected to be below the limit of quantitation of the assay (0.05 µg/mL).
Similarly, the predicted Cmax of hydrocortisone is within the range of endogenous hydrocortisone concentration (0-150 ng/mL), and therefore can not be differentiated from the endogenous cortisol.
Preclinical studies have shown that CIPRO® HC OTIC was not toxic to the guinea pig cochlea when administered intratympanically twice daily for 30 days and was only weakly irritating to rabbit skin upon repeated exposure.
Hydrocortisone has been added to aid in the resolution of the inflammatory response accompanying bacterial infection.
MicrobiologyCiprofloxacin has in vitro activity against a wide range of gram-positive and gram-negative microorganisms. The bactericidal action of ciprofloxacin results from interference with the enzyme, DNA gyrase, which is needed for the synthesis of bacterial DNA. Cross-resistance has been observed between ciprofloxacin and other fluoroquinolones. There is generally no cross-resistance between ciprofloxacin and other classes of antibacterial agents such as beta-lactams or aminoglycosides.
Ciprofloxacin has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections of acute otitis externa as described in the INDICATIONS AND USAGE section:
Aerobic gram-positive microorganism
Staphylococcus aureus
Aerobic gram-negative microorganisms
Proteus mirabilis
Pseudomonas aeruginosa
- INDICATIONS & USAGE
-
CONTRAINDICATIONS
CONTRAINDICATIONS
CIPRO® HC OTIC is contraindicated in persons with a history of hypersensitivity to hydrocortisone, ciprofloxacin or any member of the quinolone class of antimicrobial agents. This nonsterile product should not be used if the tympanic membrane is perforated. Use of this product is contraindicated in viral infections of the external canal including varicella and herpes simplex infections.
-
WARNINGS AND PRECAUTIONS
WARNINGS
NOT FOR OPHTHALMIC USE. NOT FOR INJECTION.
CIPRO® HC OTIC should be discontinued at the first appearance of a skin rash or any other sign of hypersensitivity. Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving systemic quinolones. Serious acute hypersensitivity reactions may require immediate emergency treatment.
PRECAUTIONS
GENERALAs with other antibiotic preparations, use of this product may result in overgrowth of nonsusceptible organisms, including fungi. If the infection is not improved after one week of therapy, cultures should be obtained to guide further treatment.
Information for Patients
If rash or allergic reaction occurs, discontinue use immediately and contact your physician.
Do not use in the eyes.
Avoid contaminating the dropper with material from the ear, fingers, or other sources.
Protect from light.
Shake well immediately before using.
Discard unused portion after therapy is completed.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Eight in vitro mutagenicity tests have been conducted with ciprofloxacin, and the test results are listed below:
Salmonella/Microsome Test (Negative)
E. coli DNA Repair Assay (Negative)
Mouse Lymphoma Cell Forward Mutation Assay (Positive)
Chinese Hamster V79 Cell HGPRT Test (Negative)
Syrian Hamster Embryo Cell Transformation Assay (Negative)
Saccharomyces cerevisiae Point Mutation Assay (Negative)
Saccharomyces cerevisiae Mitotic Crossover and Gene Conversion Assay (Negative)
Rat Hepatocyte DNA Repair Assay (Positive)
Thus, 2 of the 8 tests were positive, but results of the following 3 in vivo test systems gave negative results:
Rat Hepatocyte DNA Repair Assay
Micronucleus Test (Mice)
Dominant Lethal Test (Mice)
Long-term carcinogenicity studies in mice and rats have been completed for ciprofloxacin. After daily oral doses of 750 mg/kg (mice) and 250 mg/kg (rats) were administered for up to 2 years, there was no evidence that ciprofloxacin had any carcinogenic or tumorigenic effects in these species. No long term studies of CIPRO® HC OTIC suspension have been performed to evaluate carcinogenic potential.
Fertility studies performed in rats at oral doses of ciprofloxacin up to 100 mg/kg/day revealed no evidence of impairment. This would be over 1000 times the maximum recommended clinical dose of ototopical ciprofloxacin based upon body surface area, assuming total absorption of ciprofloxacin from the ear of a patient treated with CIPRO® HC OTIC twice per day.
Long term studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of topical hydrocortisone. Mutagenicity studies with hydrocortisone were negative.
Pregnancy
Teratogenic Effects
Pregnancy Category CReproduction studies have been performed in rats and mice using oral doses of up to 100 mg/kg and IV doses up to 30 mg/kg and have revealed no evidence of harm to the fetus as a result of ciprofloxacin. In rabbits, ciprofloxacin (30 and 100 mg/kg orally) produced gastrointestinal disturbances resulting in maternal weight loss and an increased incidence of abortion, but no teratogenicity was observed at either dose. After intravenous administration of doses up to 20 mg/kg, no maternal toxicity was produced in the rabbit, and no embryotoxicity or teratogenicity was observed.
Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage levels. The more potent corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
Animal reproduction studies have not been conducted with CIPRO® HC OTIC. No adequate and well controlled studies have been performed in pregnant women. Caution should be exercised when CIPRO® HC OTIC is used by a pregnant woman.
Nursing Mothers
Ciprofloxacin is excreted in human milk with systemic use. It is not known whether ciprofloxacin is excreted in human milk following topical otic administration. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric use
The safety and efficacy of CIPRO® HC OTIC have been established in pediatric patients 2 years and older (131 patients) in adequate and well-controlled clinical trials. Although no data are available on patients less than age 2 years, there are no known safety concerns or differences in the disease process in this population which would preclude use of this product in patients one year and older. See DOSAGE AND ADMINISTRATION.
-
ADVERSE REACTIONS
ADVERSE REACTIONS
In Phase 3 clinical trials, a total of 564 patients were treated with CIPRO® HC OTIC. Adverse events with at least remote relationship to treatment included headache (1.2%) and pruritus (0.4%). The following treatment-related adverse events were each reported in a single patient: migraine, hypesthesia, paresthesia, fungal dermatitis, cough, rash, urticaria, and alopecia.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION
SHAKE WELL IMMEDIATELY BEFORE USING.
For children (age 1 year and older) and adults, 3 drops of the suspension should be instilled into the affected ear twice daily for seven days. The suspension should be warmed by holding the bottle in the hand for 1-2 minutes to avoid the dizziness which may result from the instillation of a cold solution into the ear canal. The patient should lie with the affected ear upward and then the drops should be instilled. This position should be maintained for 30-60 seconds to facilitate penetration of the drops into the ear. Repeat, if necessary, for the opposite ear. Discard unused portion after therapy is completed.
-
HOW SUPPLIED
HOW SUPPLIED
CIPRO® HC OTIC is supplied as a white to off-white opaque suspension in a 10 mL bottle with a dropper dispenser.
NDC 0065-8531-10
Store below 77° F (25° C). Avoid freezing. Protect from light.
U.S. Patent Nos. 4,670,444; 4,844,902; 5,843,930; 5,965,549.
CIPRO is a registered trademark of Bayer AG.
Licensed by Bayer AG
Rx Only
Mfd. by:
ALCON CUSÍ, S.A.
08320 El Masnou - Barcelona
SPAIN
6-13-930
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CIPRO HC
ciprofloxacin hydrochloride suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:16590-053(NDC:0065-8531) Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ciprofloxacin hydrochloride (UNII: 4BA73M5E37) (ciprofloxacin - UNII:5E8K9I0O4U) ciprofloxacin hydrochloride 2 mg in 1 mL hydrocortisone (UNII: WI4X0X7BPJ) (hydrocortisone - UNII:WI4X0X7BPJ) hydrocortisone 10 mg in 1 mL benzyl alcohol (UNII: LKG8494WBH) (benzyl alcohol - UNII:LKG8494WBH) benzyl alcohol 9 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16590-053-10 10 mL in 1 BOTTLE, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020805 02/10/1998 Labeler - Stat Rx USA (786036330)


