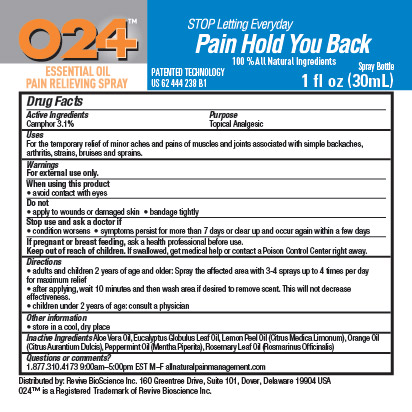
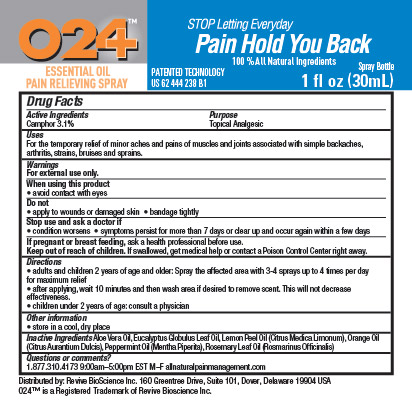
Label: O24 ESSENTIAL OIL PAIN RELIEVING- camphor spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 76433-004-30 - Packager: Revive Bioscience, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 26, 2012
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- DO NOT USE
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- adults and children 2 years of age and older: Spray the affected area with 3-4 sprays up to 4 times per day for maximum relief
- after applying, wait 10 minutes and then wash area if desire to remove scent. This will not decrease effectiveness.
- children under 2 years of age: consult a physician
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
O24 ESSENTIAL OIL PAIN RELIEVING
camphor sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76433-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 1 mL in 30 mL Inactive Ingredients Ingredient Name Strength EUCALYPTUS OIL (UNII: 2R04ONI662) LEMON OIL (UNII: I9GRO824LL) ORANGE OIL (UNII: AKN3KSD11B) PEPPERMINT OIL (UNII: AV092KU4JH) ROSEMARY OIL (UNII: 8LGU7VM393) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76433-004-30 30 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 02/01/2012 Labeler - Revive Bioscience, Inc. (248262920) Registrant - Revive Bioscience, Inc. (248262920) Establishment Name Address ID/FEI Business Operations Sigan Industries 255106239 manufacture