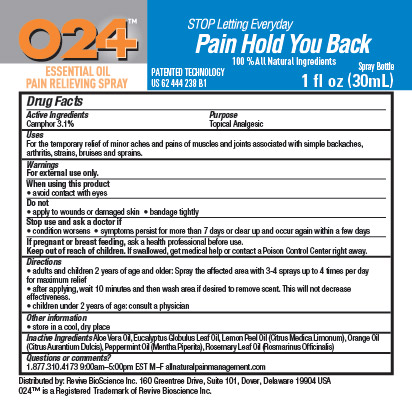
O24 ESSENTIAL OIL PAIN RELIEVING- camphor spray
Revive Bioscience, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
For the temporary relief of minor aches and pains of muscles and joints associated with simple backaches, arthritis, strains, bruises and sprains.
Do not
- apply to wounds or damaged skin
- bandage tightly
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
If pregnant or breast feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 years of age and older: Spray the affected area with 3-4 sprays up to 4 times per day for maximum relief
- after applying, wait 10 minutes and then wash area if desire to remove scent. This will not decrease effectiveness.
- children under 2 years of age: consult a physician
store in a cool, dry place
Aloe Vera Oil, Eucalyptus Globulus Leaf Oil, Lemon Peel Oil (Citrus Medica Limonum), Orange Oil (Citrus Aurantium Dulcis), Peppermint Oil (Mentha Piperita), Rosemary Leaf Oil (Rosmarinus Officinalis)
Questions or comments?
1.877.310.4173 9:00am-5:00pm EST M-F
allnaturalpainmanagement.com
Revive Bioscience, Inc.