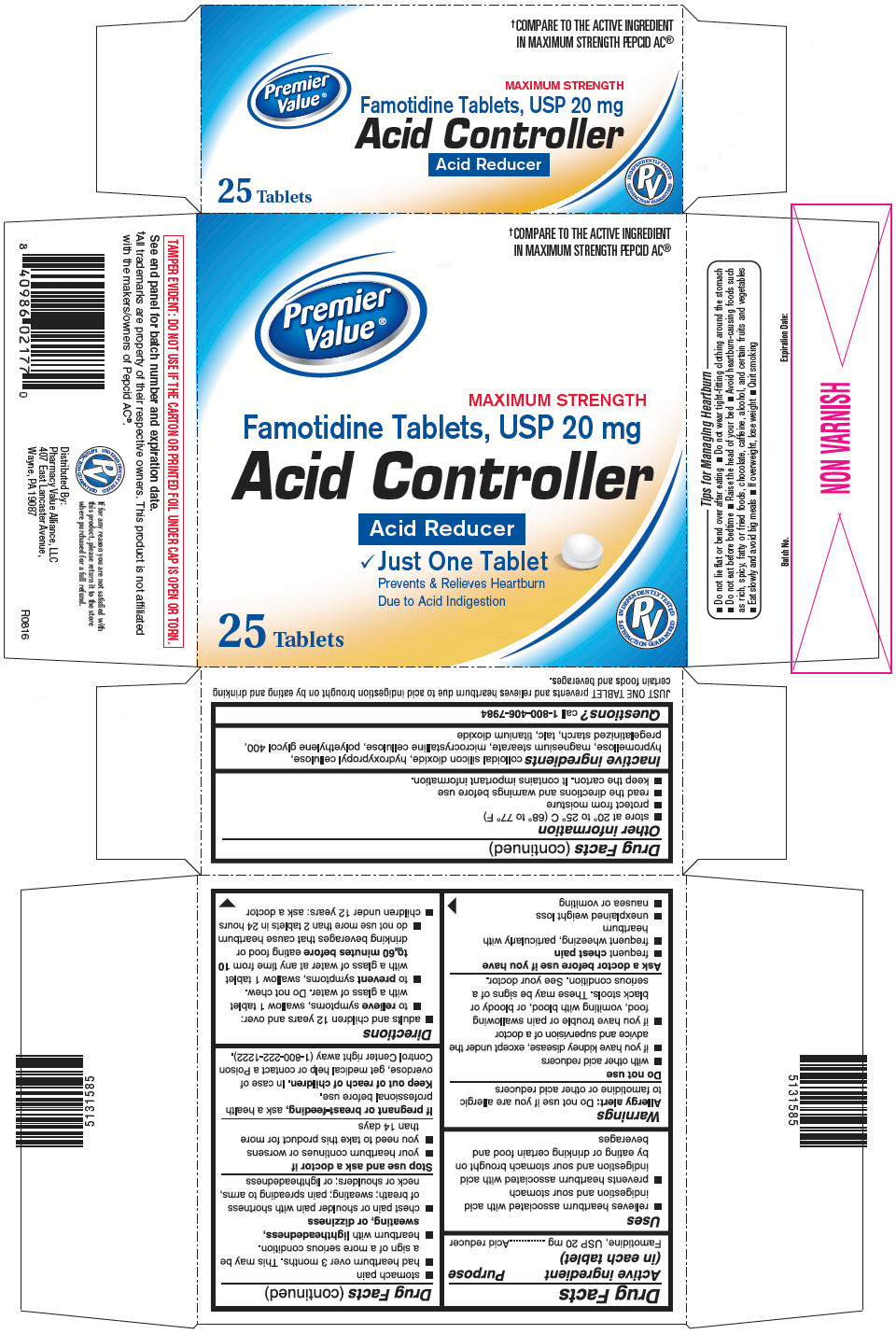
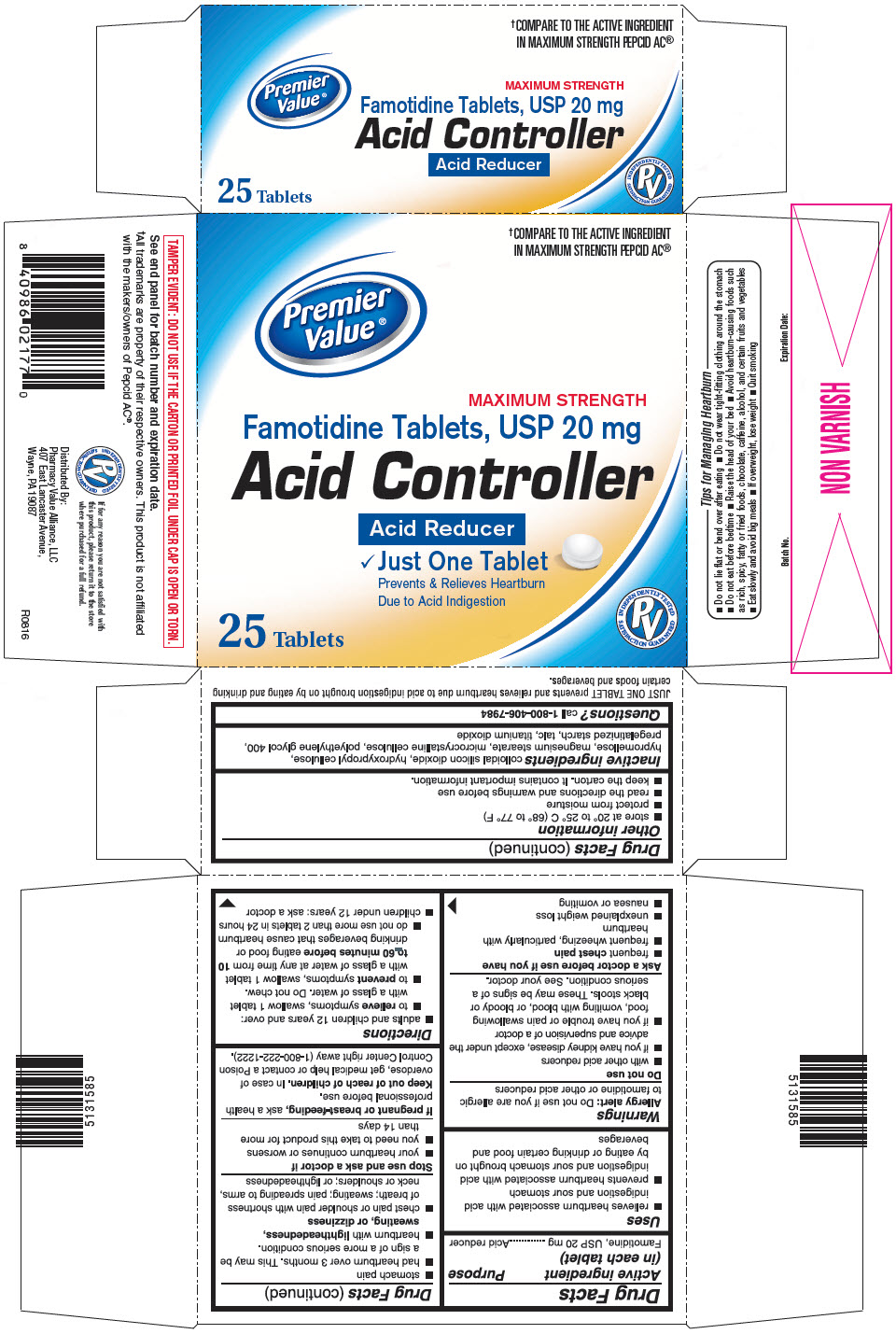
Label: PREMIER VALUE FAMOTIDINE- famotidine tablet, film coated
- NDC Code(s): 68016-008-01, 68016-010-81, 68016-010-82
- Packager: Premier Value
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 27, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
- with other acid reducers
- if you have kidney disease, except under the advice and supervision of a doctor
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating, or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
-
Directions
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water. Do not chew.
- to prevent symptoms, swallow 1 tablet with a glass of water at any time from 10 to 60 minutes before eating food or drinking beverages that cause heartburn
- do not use more than 2 tablets in 24 hours
- children under 12 years: ask a doctor
- adults and children 12 years and over:
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 25 Tablet Bottle Carton
- PRINCIPAL DISPLAY PANEL - 25 Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
PREMIER VALUE FAMOTIDINE
famotidine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68016-010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FAMOTIDINE (UNII: 5QZO15J2Z8) (FAMOTIDINE - UNII:5QZO15J2Z8) FAMOTIDINE 10 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color pink Score no score Shape ROUND Size 8mm Flavor Imprint Code 035 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68016-010-81 30 in 1 BLISTER PACK; Type 0: Not a Combination Product 06/25/2010 2 NDC:68016-010-82 60 in 1 BOTTLE; Type 0: Not a Combination Product 06/25/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090283 06/25/2010 PREMIER VALUE FAMOTIDINE
famotidine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68016-008 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FAMOTIDINE (UNII: 5QZO15J2Z8) (FAMOTIDINE - UNII:5QZO15J2Z8) FAMOTIDINE 20 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape ROUND Size 8mm Flavor Imprint Code 036 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68016-008-01 25 in 1 BOTTLE; Type 0: Not a Combination Product 07/23/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090283 07/23/2010 Labeler - Premier Value (101668460) Registrant - Sun Pharmaceutical Industries Inc. (146974886) Establishment Name Address ID/FEI Business Operations Ohm Laboratories Inc. 184769029 manufacture(68016-010, 68016-008)