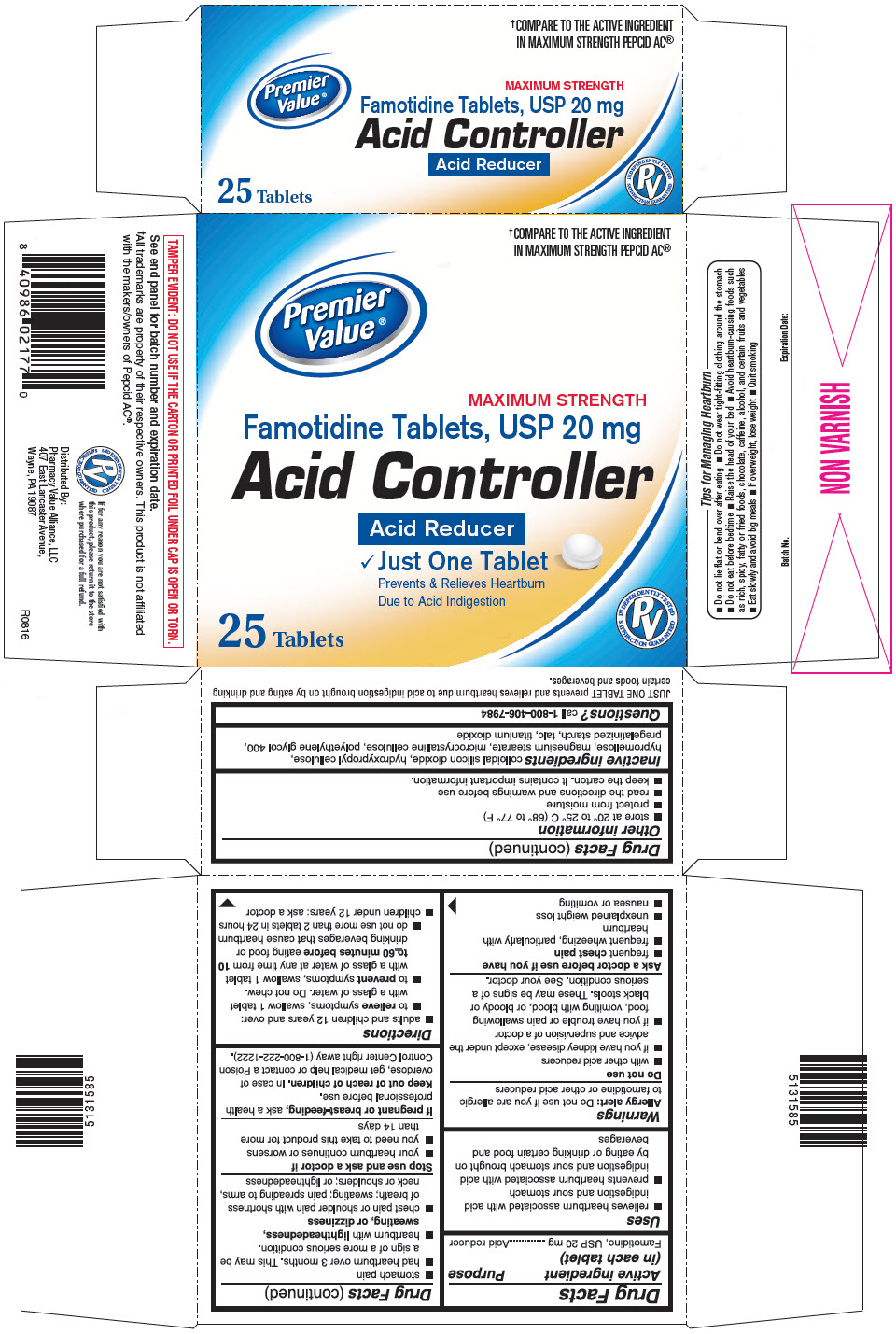
Uses
- relieves heartburn associated with acid indigestion and sour stomach
- prevents heartburn associated with acid indigestion and sour stomach brought on by eating or drinking certain food and beverages
Warnings
Do not use
- with other acid reducers
- if you have kidney disease, except under the advice and supervision of a doctor
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating, or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
Directions
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water. Do not chew.
- to prevent symptoms, swallow 1 tablet with a glass of water at any time from 10 to 60 minutes before eating food or drinking beverages that cause heartburn
- do not use more than 2 tablets in 24 hours
- children under 12 years: ask a doctor
Other information
- store at 20° to 25° C (68° to 77° F)
- protect from moisture
- read the directions and warnings before use
- keep the carton. It contains important information.
Inactive ingredients
colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol 400, pregelatinized starch, talc, titanium dioxide
PRINCIPAL DISPLAY PANEL - 25 Tablet Bottle Carton
†COMPARE TO THE ACTIVE INGREDIENT
IN MAXIMUM STRENGTH PEPCID AC®
Premier
Value ®
MAXIMUM STRENGTH
Famotidine Tablets, USP 20 mg
Acid Controller
Acid Reducer
- ✓
- Just One Tablet
Prevents & Relieves Heartburn
Due to Acid Indigestion
25 Tablets
INDEPENDENTLY TESTED
PV
SATISFACTION GUARANTEED