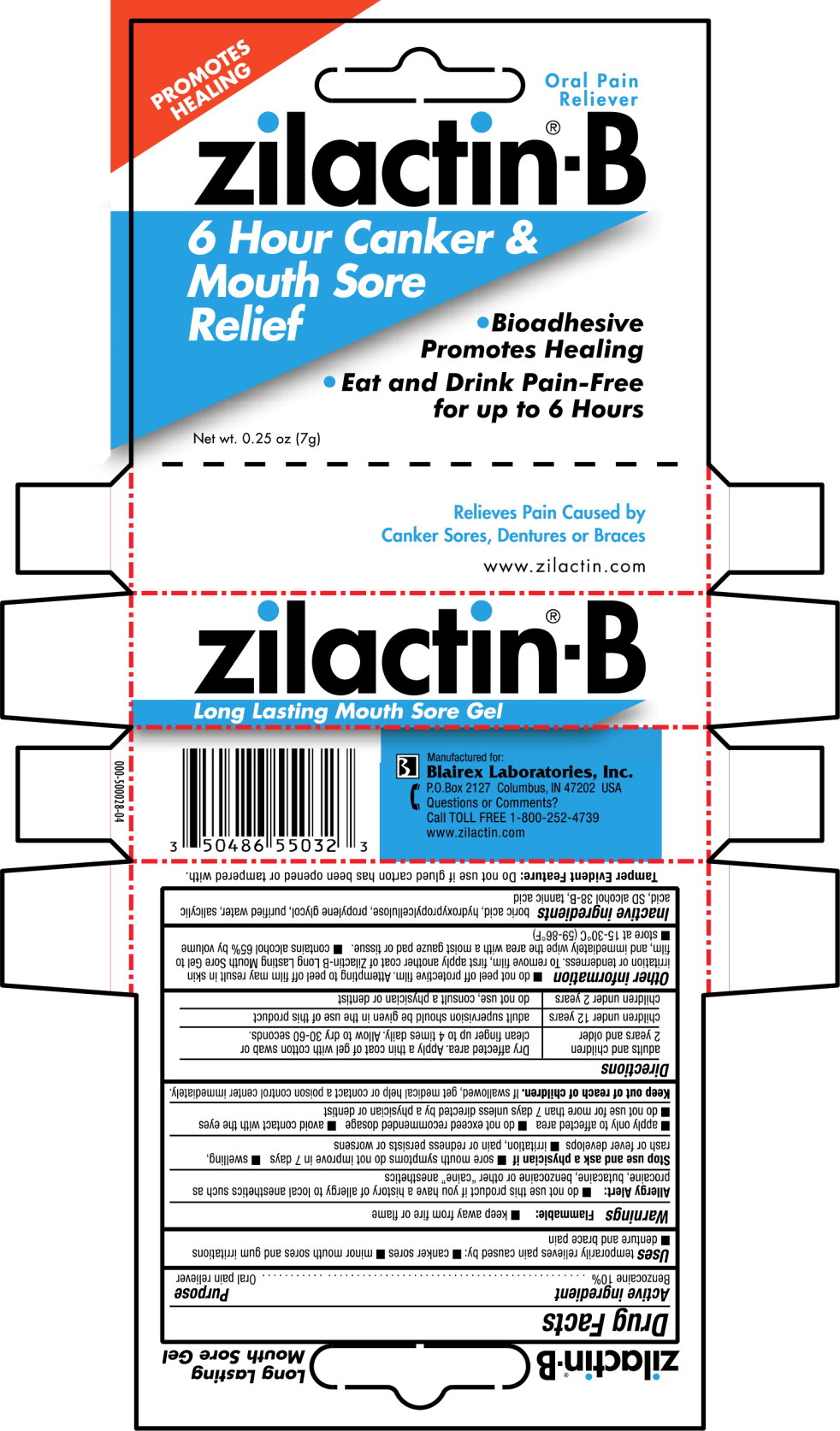
Label: ZILACTIN-B- benzocaine gel
- NDC Code(s): 50486-550-32
- Packager: Blairex Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 23, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
Allergy Alert:
- do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other “caine” anesthetics
Stop use and ask a physician if
- sore mouth symptoms do not improve in 7 days
- swelling, rash or fever develops
- irritation, pain or redness persists or worsens
- apply only to affected area
- do not exceed recommended dosage
- avoid contact with the eyes
- do not use for more than 7 days unless directed by a physician or dentist
-
Directions
adults and children
2 years and olderDry affected area. Apply a thin coat of gel with cotton swab or clean finger up to 4 times daily. Allow to dry 30-60 seconds. children under 12 years adult supervision should be given in the use of this product children under 2 years do not use, consult a physician or dentist -
Other information
- do not peel off protective film. Attempting to peel off film may result in skin irritation or tenderness. To remove film, first apply another coat of Zilactin-B Long Lasting Mouth Sore Gel to film, and immediately wipe the area with a moist gauze pad or tissue.
- contains alcohol 65% by volume
- store at 15-30°C (59-86°F)
- Inactive ingredients
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ZILACTIN-B
benzocaine gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50486-550 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) HYDROXYPROPYL CELLULOSE (TYPE M) (UNII: U3JF91U133) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SALICYLIC ACID (UNII: O414PZ4LPZ) ALCOHOL (UNII: 3K9958V90M) PEPPERMINT OIL (UNII: AV092KU4JH) TANNIC ACID (UNII: 28F9E0DJY6) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50486-550-32 1 in 1 CARTON 06/29/2005 1 7 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 06/29/2005 Labeler - Blairex Laboratories, Inc. (092575133)

 Questions or Comments?
Questions or Comments?