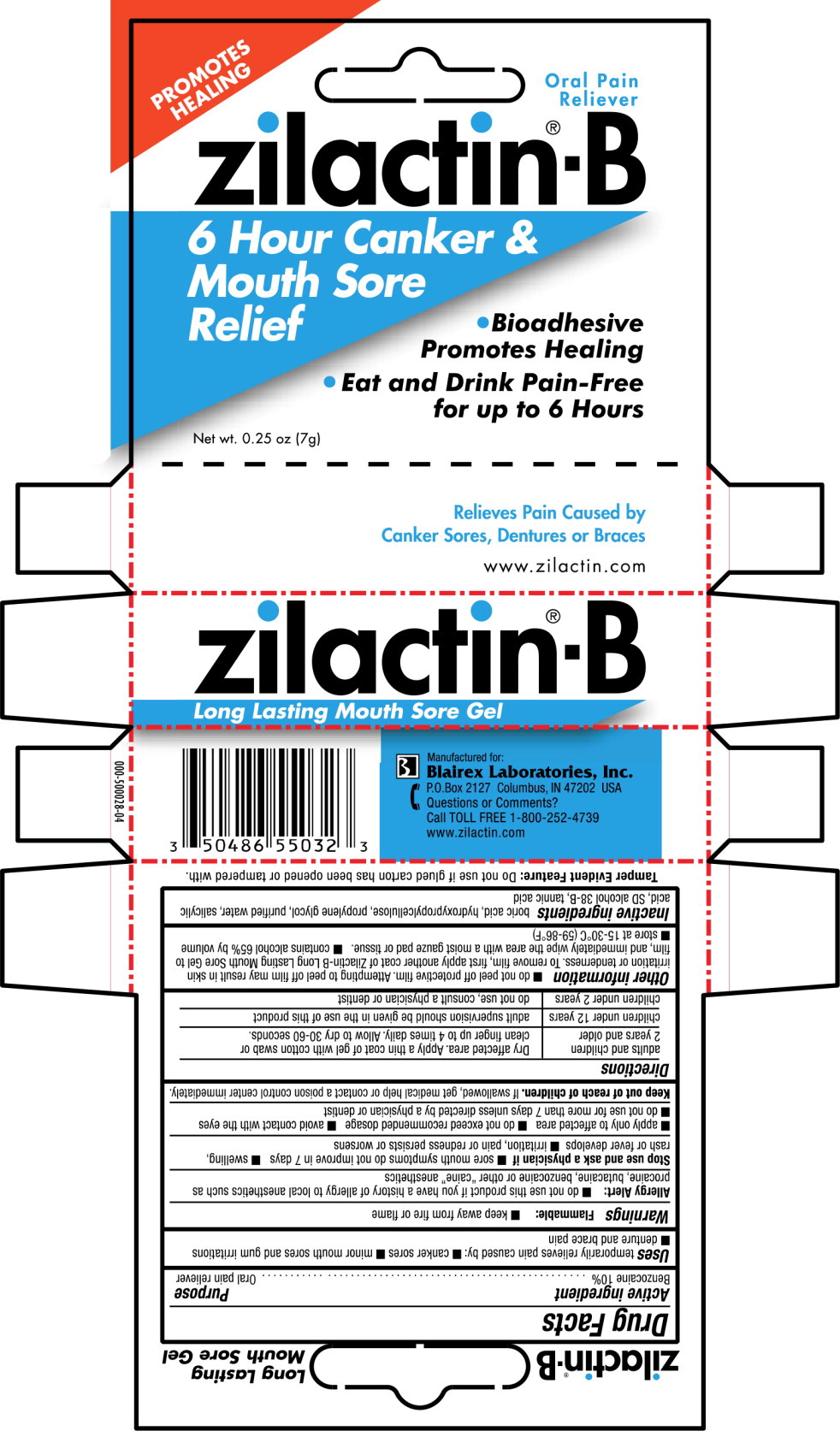
Use
temporarily relieves pain caused by:
- canker sores
- minor mouth sores and gum irritations
- denture and brace pain
Warnings
Allergy Alert:
- do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other “caine” anesthetics
Stop use and ask a physician if
- sore mouth symptoms do not improve in 7 days
- swelling, rash or fever develops
- irritation, pain or redness persists or worsens
- apply only to affected area
- do not exceed recommended dosage
- avoid contact with the eyes
- do not use for more than 7 days unless directed by a physician or dentist
Directions
| adults and children
2 years and older | Dry affected area. Apply a thin coat of gel with cotton swab or clean finger up to 4 times daily. Allow to dry 30-60 seconds. |
| children under 12 years | adult supervision should be given in the use of this product |
| children under 2 years | do not use, consult a physician or dentist |
Other information
- do not peel off protective film. Attempting to peel off film may result in skin irritation or tenderness. To remove film, first apply another coat of Zilactin-B Long Lasting Mouth Sore Gel to film, and immediately wipe the area with a moist gauze pad or tissue.
- contains alcohol 65% by volume
- store at 15-30°C (59-86°F)
 Questions or Comments?
Questions or Comments?