Label: CLEAN FORCE- ethyl alcohol solution
- NDC Code(s): 47593-406-41, 47593-406-80
- Packager: Ecolab Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
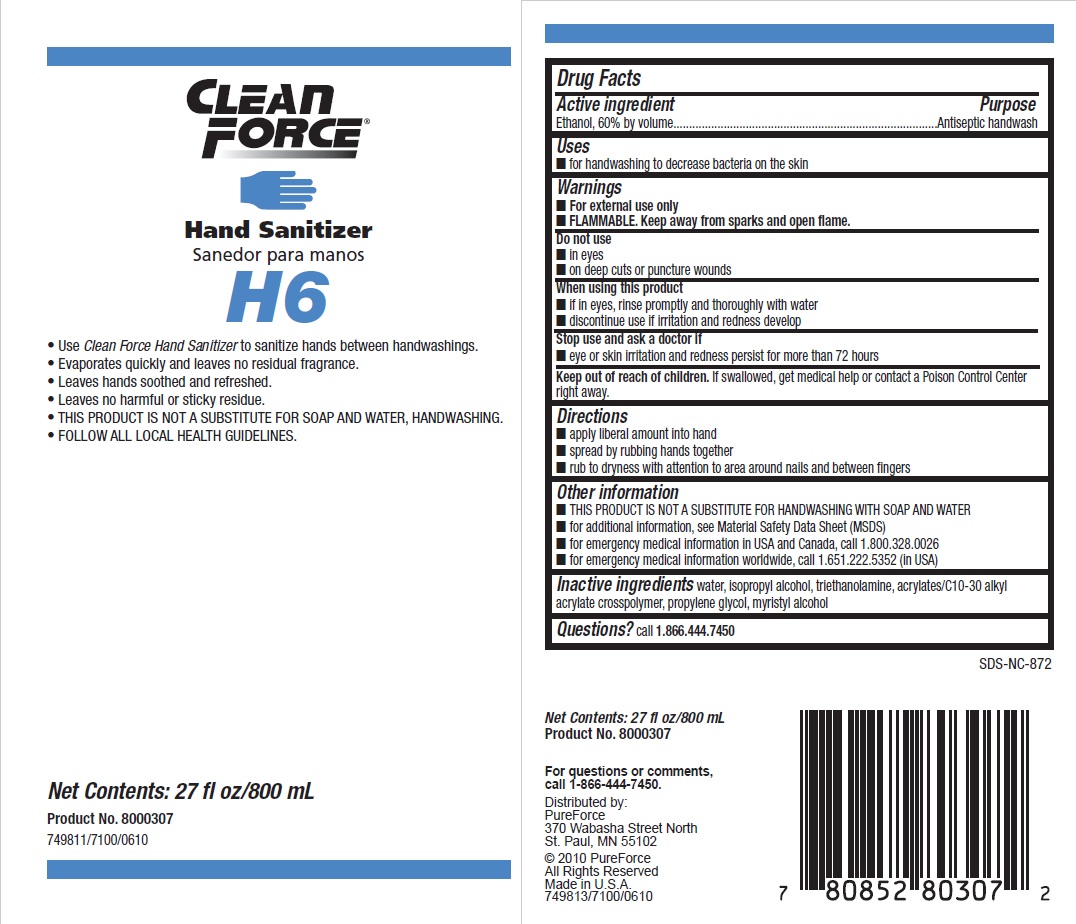
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- INACTIVE INGREDIENT
- QUESTIONS
-
Principal display panel and representative label
CLEAN FORCE
HAND SANITIZER
Use Clean Force Hand Sanitizer to sanitize hands between handwashings.
Evaporates quickly and leaves no residual fragrance.
Leaves hands soothed and refreshed.
Leaves no harmful or sticky residue.
THIS PRODUCT IS NOT A SUBSTITUTE FOR SOAP AND WATER, HANDWASHING.
FOLLOW ALL LOCAL HEALTH GUIDELINES.
Net Contents: 27 fl oz/800 mL
Product No. 8000307749811/7100/0610
Distributed by:
PureForce
370 Wabasha Street North
St. Paul, MN 55102
© 2010 PureForce
All Rights Reserved
Made in U.S.A.
749813/7100/0610
-
INGREDIENTS AND APPEARANCE
CLEAN FORCE
ethyl alcohol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47593-406 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 60 mL in 100 mL Inactive Ingredients Ingredient Name Strength CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 809Y72KV36) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) MYRISTYL ALCOHOL (UNII: V42034O9PU) ISOPROPYL ALCOHOL (UNII: ND2M416302) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47593-406-41 750 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/29/2013 02/19/2024 2 NDC:47593-406-80 800 mL in 1 POUCH; Type 0: Not a Combination Product 10/27/2003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 10/27/2003 Labeler - Ecolab Inc. (006154611)