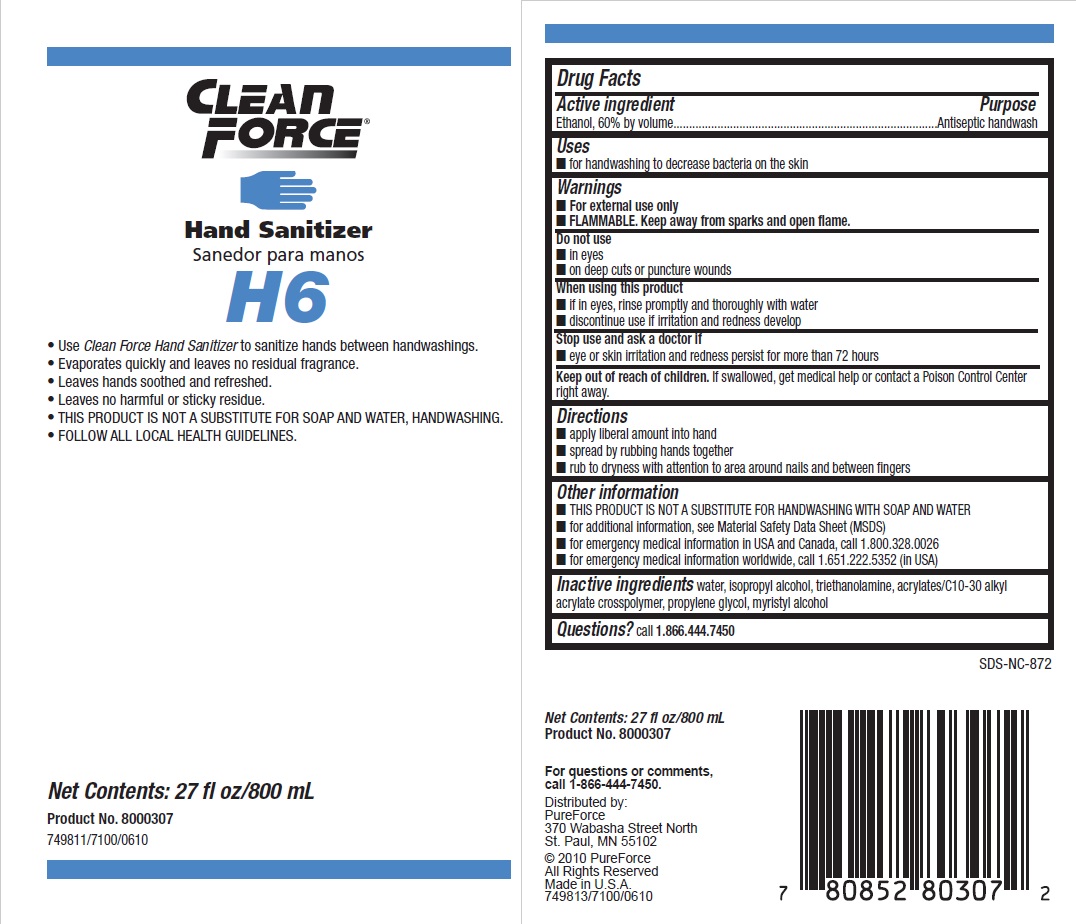
Warnings
- For external use only
- Flammable, keep away from sparks and open flame
Directions
- Apply liberal amount into hand
- Spread by rubbing hands together
- Rub to dryness with attention to area around nails and between fingers
Other information
- THIS PRODUCT IS NOT A SUBSTITUTE FOR HANDWASHING WITH SOAP AND WATER
- for additional information, see Material Safety Data Sheet (MSDS)
- for emergency medical information in USA and Canada, call 1.800.328.0026
- for emergency medical information worldwide, call 1.651.222.5352 (in USA)
Inactive ingredients water, isopropyl alcohol, triethanolamine, acrylates/C10-30 alkyl acrylate crosspolymer, propylene glycol, myristyl alcohol
Principal display panel and representative label
CLEAN FORCE
HAND SANITIZER
Use Clean Force Hand Sanitizer to sanitize hands between handwashings.
Evaporates quickly and leaves no residual fragrance.
Leaves hands soothed and refreshed.
Leaves no harmful or sticky residue.
THIS PRODUCT IS NOT A SUBSTITUTE FOR SOAP AND WATER, HANDWASHING.
FOLLOW ALL LOCAL HEALTH GUIDELINES.
Net Contents: 27 fl oz/800 mL
Product No. 8000307749811/7100/0610
Distributed by:
PureForce
370 Wabasha Street North
St. Paul, MN 55102
© 2010 PureForce
All Rights Reserved
Made in U.S.A.
749813/7100/0610