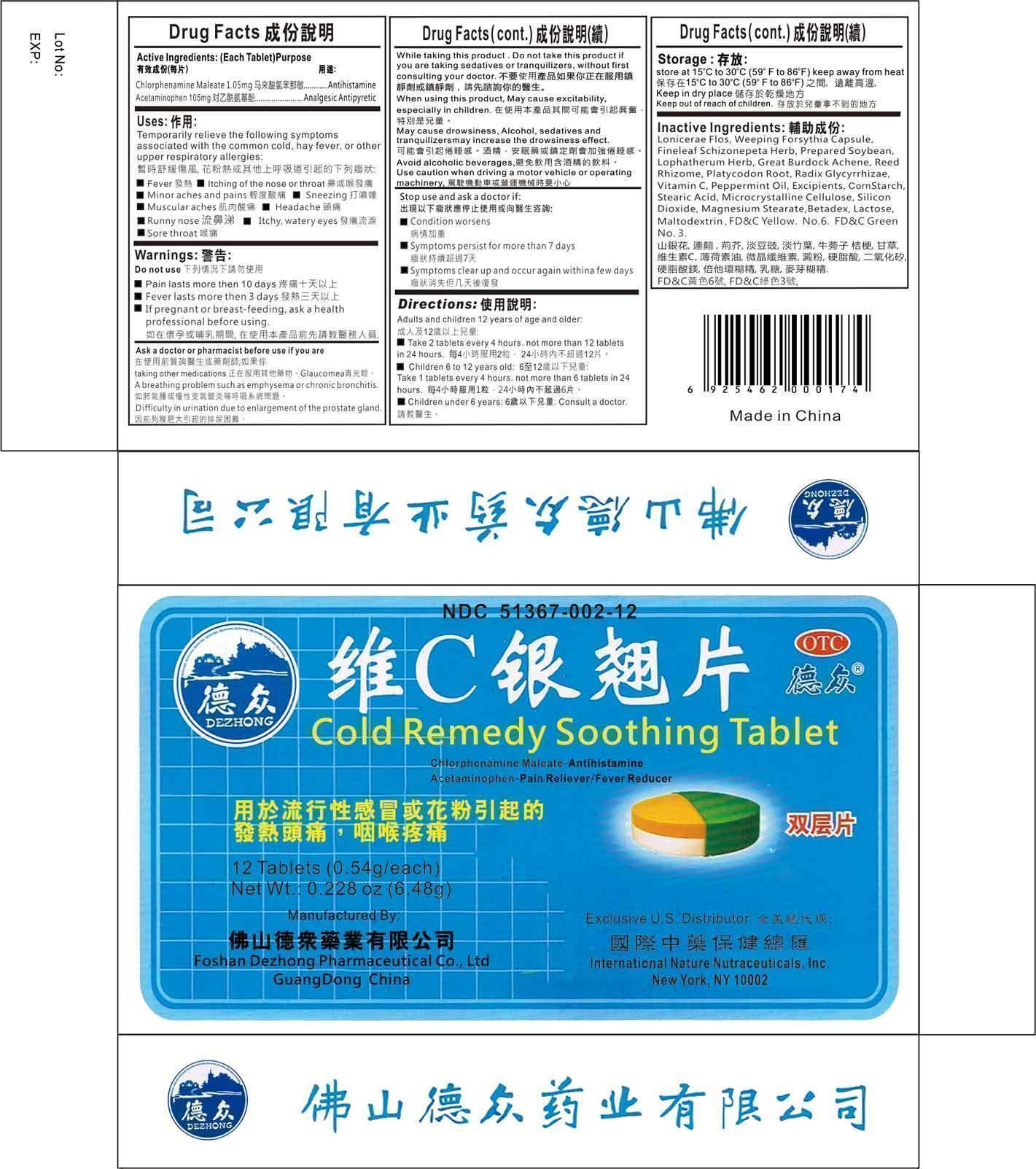
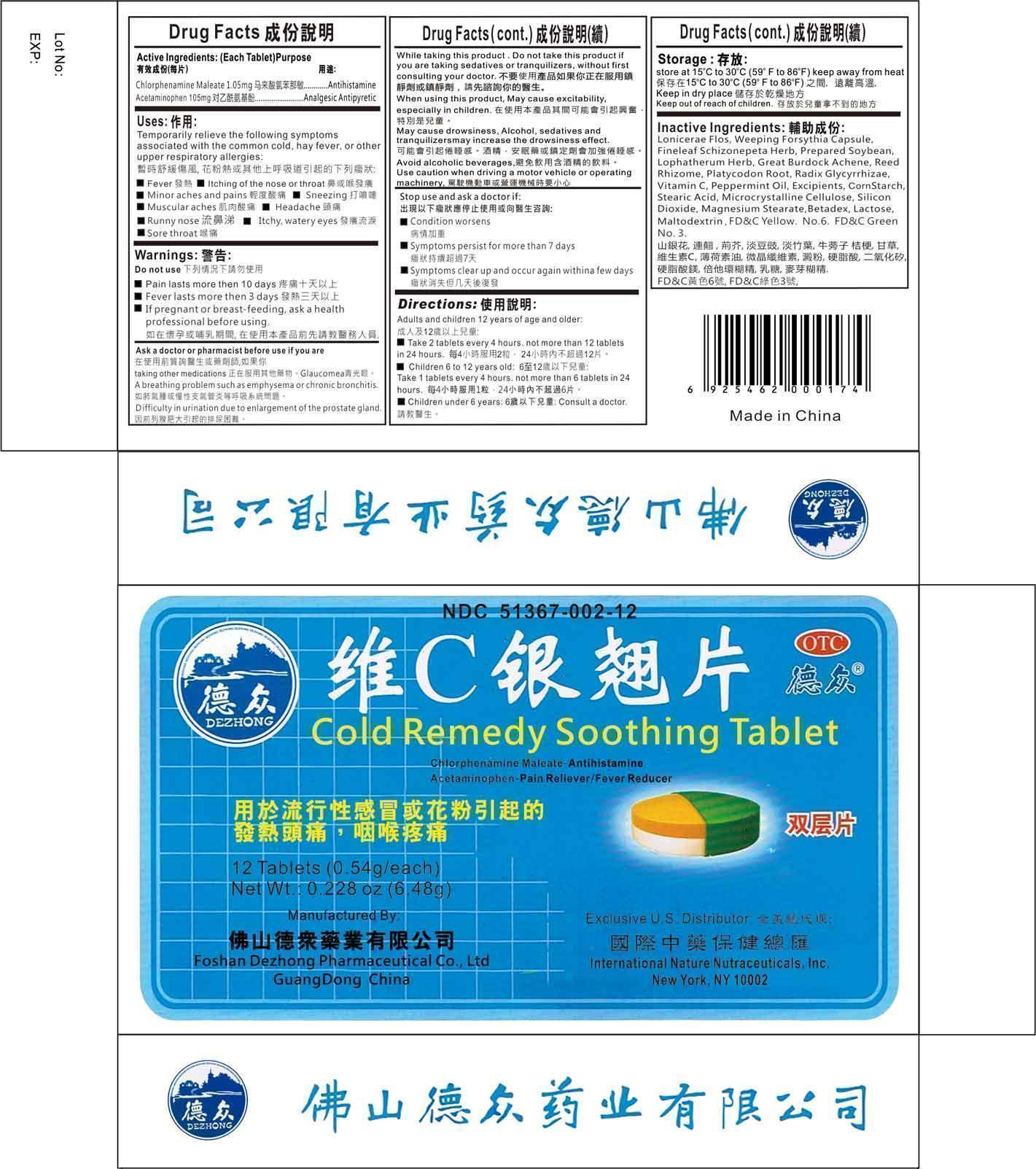
Label: COLD REMEDY SOOTHING- chlorpheniramine maleate, acetaminophen tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 51367-002-12 - Packager: International Nature Nutraceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 1, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Uses
- Warnings
- Ask a doctor or pharmacist before use if you are
-
While taking this product
Do not use this product if you are taking sedatives or tranquilizers, without first consulting your doctor.
When using this product, may cause excitability, especially in children
May cause drowsiness. Alcohol, sedatives, and tranquilizers may increase the drowsiness effect.
Avoid alcoholic beverages
Use caution when driving a motor vehicle or operating machinery.
- Stop use and doctor if
- Directions
- Storage
- Keep out of reach of children
-
Inactive Ingredients
Lonicerae Flos, Weeping Forsythia Capsule, Fineleaf Schizonepeta Herb, Prepared Soybean, Lophatherum Herb, Great Burdock Achene, Reed Rhizome, Platycodon Root, Radix Glycyrrhizae, Vitamin C, Peppermint Oil.
Excipients:
Corn Starch, Stearic Acid, Microcrystalline Cellulose, Silicon Dioxide, Magnesium Stearate, Betadex, Lactose, Maltodextrin, FD&C Yellow No. 6, FD&C Green No. 3
- Image
-
INGREDIENTS AND APPEARANCE
COLD REMEDY SOOTHING
chlorpheniramine maleate, acetaminophen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51367-002 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE 1.05 mg ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 105 mg Inactive Ingredients Ingredient Name Strength LONICERA CONFUSA WHOLE (UNII: 22L2C7GWOA) FORSYTHIA SUSPENSA WHOLE (UNII: RO2K2K561Z) SCHIZONEPETA TENUIFOLIA FLOWERING TOP (UNII: 2FN3BA1MZE) SOYBEAN (UNII: L7HT8F1ZOD) LOPHATHERUM GRACILE LEAF (UNII: R417138GE9) ARCTIUM LAPPA FRUIT (UNII: EA541308MV) PHRAGMITES AUSTRALIS ROOT (UNII: BBJ7AOM815) PLATYCODON GRANDIFLORUM ROOT (UNII: 2DF0NS0O2Z) GLYCYRRHIZA URALENSIS (UNII: 42B5YD8F0K) ASCORBIC ACID (UNII: PQ6CK8PD0R) PEPPERMINT OIL (UNII: AV092KU4JH) MODIFIED CORN STARCH (1-OCTENYL SUCCINIC ANHYDRIDE) (UNII: 461P5CJN6T) STEARIC ACID (UNII: 4ELV7Z65AP) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) BETADEX (UNII: JV039JZZ3A) LACTOSE (UNII: J2B2A4N98G) MALTODEXTRIN (UNII: 7CVR7L4A2D) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) Product Characteristics Color green Score no score Shape ROUND Size 11mm Flavor LICORICE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51367-002-12 12 in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 02/24/1987 Labeler - International Nature Nutraceuticals (006106879) Establishment Name Address ID/FEI Business Operations Foshan Dezhong Pharmaceutical Co. Ltd 529754442 manufacture(51367-002)