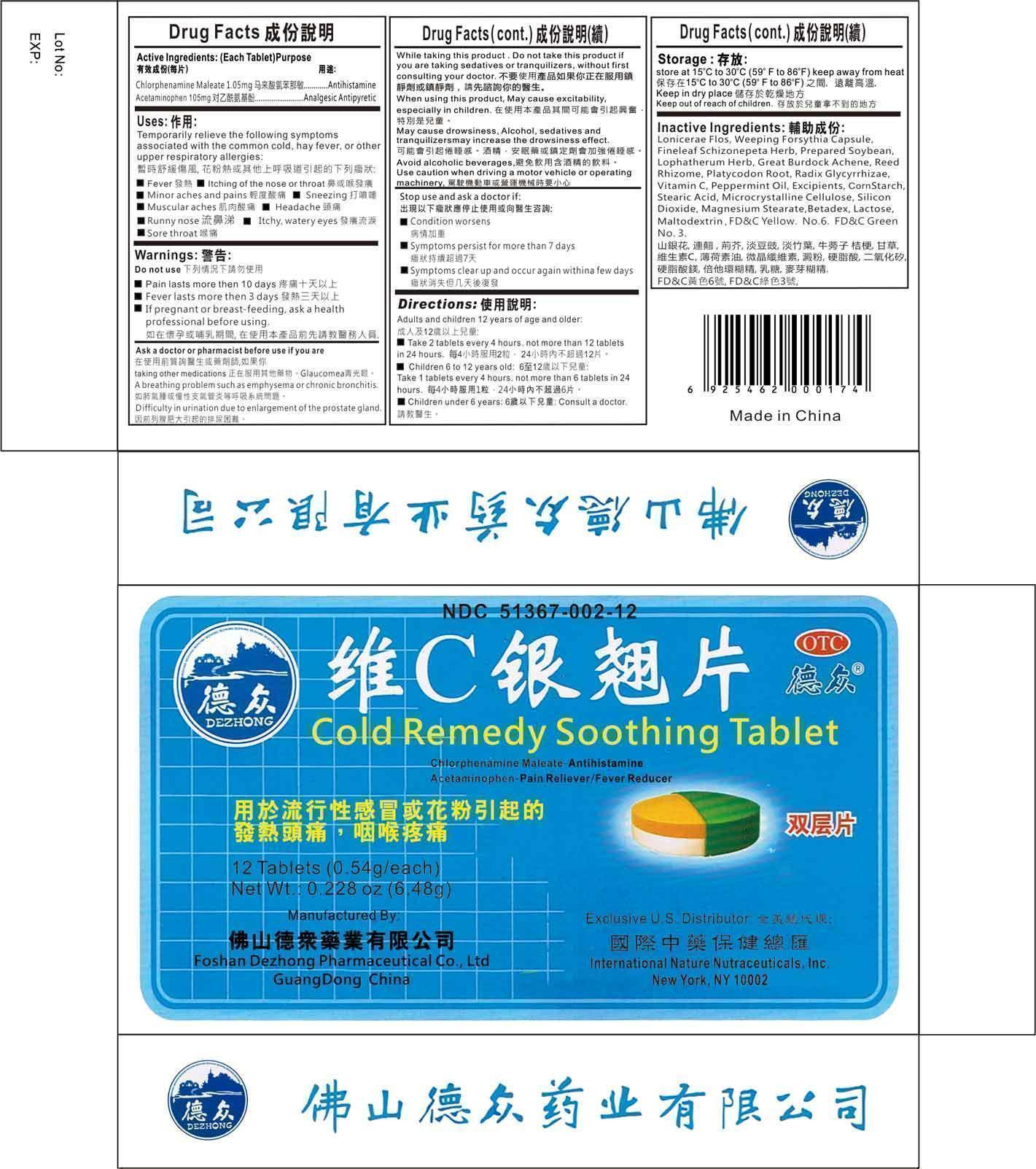
Uses
Temporarily relieve the following symptoms associated with the common cold, hay fever, or other upper respiratory allergies:
- Fever
- Itiching of the nose or throat
- Minor aches and pains
- Sneezing
- Muscle aches
- Headache
- Runny nose
- Itchy eyes
Warnings
Do Not Use:
- Pain lasts more than 10 days
- Fever lasts more than 3 days
- If pregnant or breast-feeding, ask a health professional before using
Ask a doctor or pharmacist before use if you are
- Taking other medications
- Have glaucomea
- A breathing problem such as emphysema or chronic bronchitis
- Difficulty in urination due to enlargement of the prostate gland
While taking this product
Do not use this product if you are taking sedatives or tranquilizers, without first consulting your doctor.
When using this product, may cause excitability, especially in children
May cause drowsiness. Alcohol, sedatives, and tranquilizers may increase the drowsiness effect.
Avoid alcoholic beverages
Use caution when driving a motor vehicle or operating machinery.
Stop use and doctor if
- Condition worsens
- Symptoms persit for more than 7 days
- Symptoms clear up and occur again within a few days
Directions
Adults and children 12 years of age and older
- Take 2 tablets every 4 hours, not to exceed more than 12 tablets in 24 hours
Children ages 6 to 12 years old
- Take 1 tablet every 4 hours, not to exceed more than 6 tablets in 24 hours
Children under 6 years of age
- Consult a doctor
Inactive Ingredients
Lonicerae Flos, Weeping Forsythia Capsule, Fineleaf Schizonepeta Herb, Prepared Soybean, Lophatherum Herb, Great Burdock Achene, Reed Rhizome, Platycodon Root, Radix Glycyrrhizae, Vitamin C, Peppermint Oil.
Excipients:
Corn Starch, Stearic Acid, Microcrystalline Cellulose, Silicon Dioxide, Magnesium Stearate, Betadex, Lactose, Maltodextrin, FD&C Yellow No. 6, FD&C Green No. 3