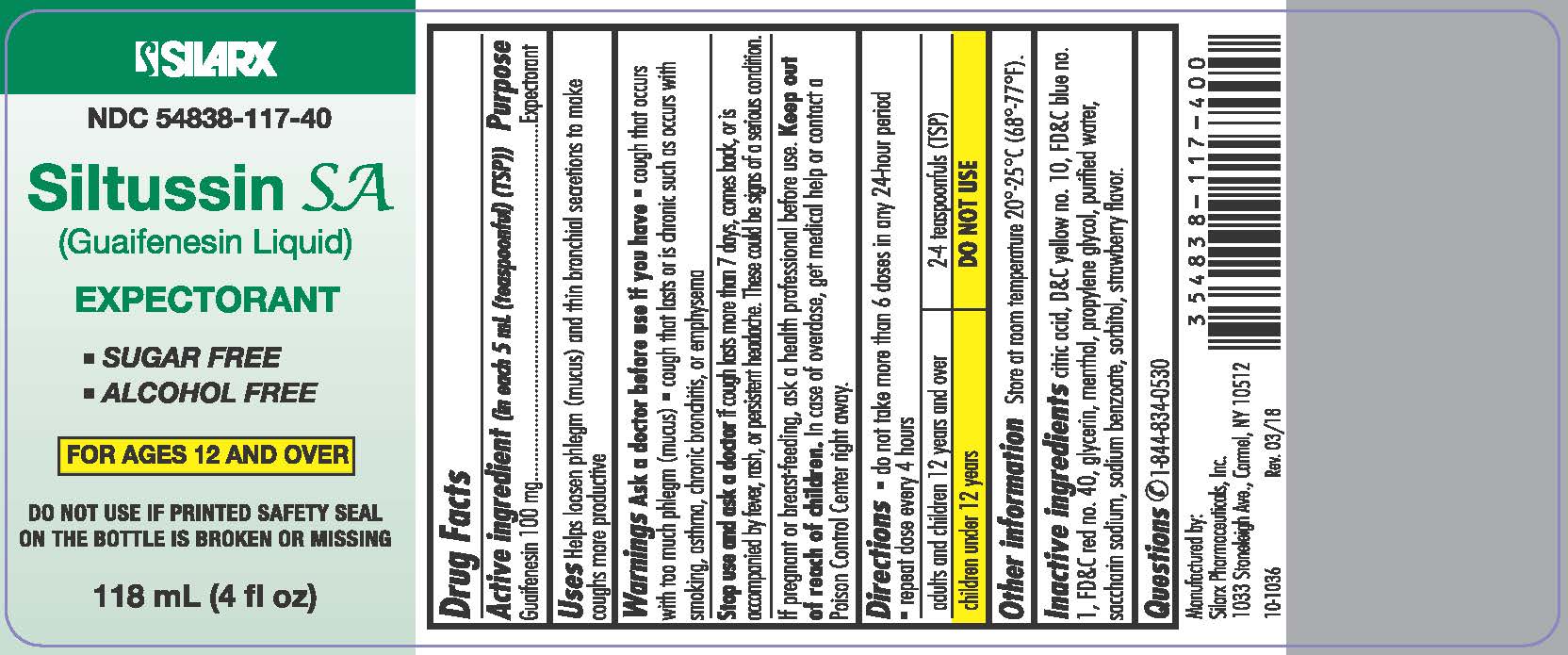
Label: SILTUSSIN SA- guaifenesin liquid
- NDC Code(s): 54838-117-40, 54838-117-70, 54838-117-80
- Packager: Lannett Company, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 11, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
Warnings
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough that occurs with too much phlegm (mucus)
-
Directions
- do not take more than 6 doses in any 24-hour period
- repeat dose every 4 hours
adults and children 12 years and over
2-4 teaspoonfuls (TSP)
children under 12 years DO NOT USE Other information
Store at room temperature 20°-25°C (68°-77°F). Do not accept if imprinted tamper evident safety seal around cap is broken or missing. - do not take more than 6 doses in any 24-hour period
- Inactive ingredients
- Questions
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SILTUSSIN SA
guaifenesin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54838-117 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) MENTHOL (UNII: L7T10EIP3A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) Product Characteristics Color Score Shape Size Flavor STRAWBERRY (strawberry flavor) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54838-117-40 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/05/1998 12/31/2024 2 NDC:54838-117-70 237 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/05/1998 09/30/2024 3 NDC:54838-117-80 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/05/1998 11/30/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 10/05/1998 12/31/2024 Labeler - Lannett Company, Inc. (002277481)