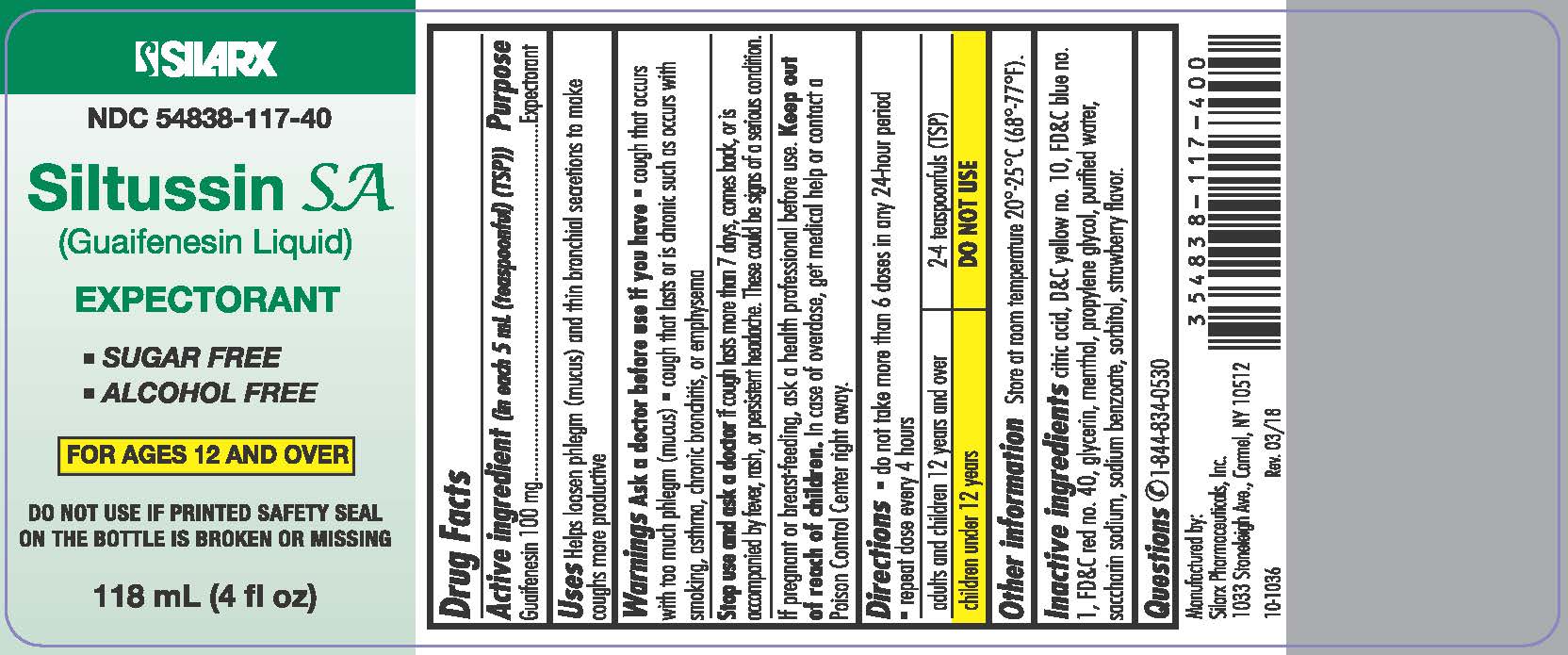
SILTUSSIN SA- guaifenesin liquid
Lannett Company, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient: Guaifenesin 100 mg (in each 5 mL (teaspoon)(TSP))
Uses Helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
Warnings
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Stop use and ask a doctor if cough lasts more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- do not take more than 6 doses in any 24-hour period
- repeat dose every 4 hours
adults and children 12 years and over
| 2-4 teaspoonfuls (TSP)
|
| children under 12 years | DO NOT USE |
Other information
Store at room temperature 20°-25°C (68°-77°F). Do not accept if imprinted tamper evident safety seal around cap is broken or missing.
Inactive ingredients
citric acid, D&C yellow no. 10, FD&C blue no. 1, FD&C red no. 40, glycerin, menthol, propylene glycol, purified water, saccharin sodium, sodium benzoate, sorbitol, strawberry flavor.
Manufactured by:
Silarx Pharmaceuticals, Inc.
1033 Stoneleigh Ave., Carmel, NY 10512
Lannett Company, Inc.