Label: METOCLOPRAMIDE- metoclopramide hydrochloride injection
-
NDC Code(s):
23155-240-31,
23155-240-32,
23155-240-33,
23155-240-41, view more23155-240-42, 23155-240-43
- Packager: Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
BOXED WARNING
Treatment with metoclopramide can cause tardive dyskinesia, a serious movement disorder that is often irreversible. The risk of developing tardive dyskinesia increases with duration of treatment and total cumulative dose.
Metoclopramide therapy should be discontinued in patients who develop signs or symptoms of tardive dyskinesia. There is no known treatment for tardive dyskinesia. In some patients, symptoms may lessen or resolve after metoclopramide treatment is stopped.
Treatment with metoclopramide for longer than 12 weeks should be avoided in all but rare cases where therapeutic benefit is thought to outweigh the risk of developing tardive dyskinesia. See WARNINGS.
-
DESCRIPTION
Metoclopramide hydrochloride is a white crystalline, odorless substance, freely soluble in water. Chemically, it is 4-amino-5-chloro-N-[2-(diethylamino)ethyl]-2-methoxy benzamide monohydrochloride monohydrate. Molecular weight: 354.3.
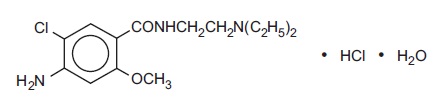
Metoclopramide Injection, USP is a clear, colorless, sterile solution with a pH of 2.5 to 6.5 for intravenous (IV) or intramuscular (IM) administration.
This product is light sensitive. It should be inspected before use and discarded if either color or particulate is observed.
2 mL single dose vials; 10 mL and 30 mL single dose vials
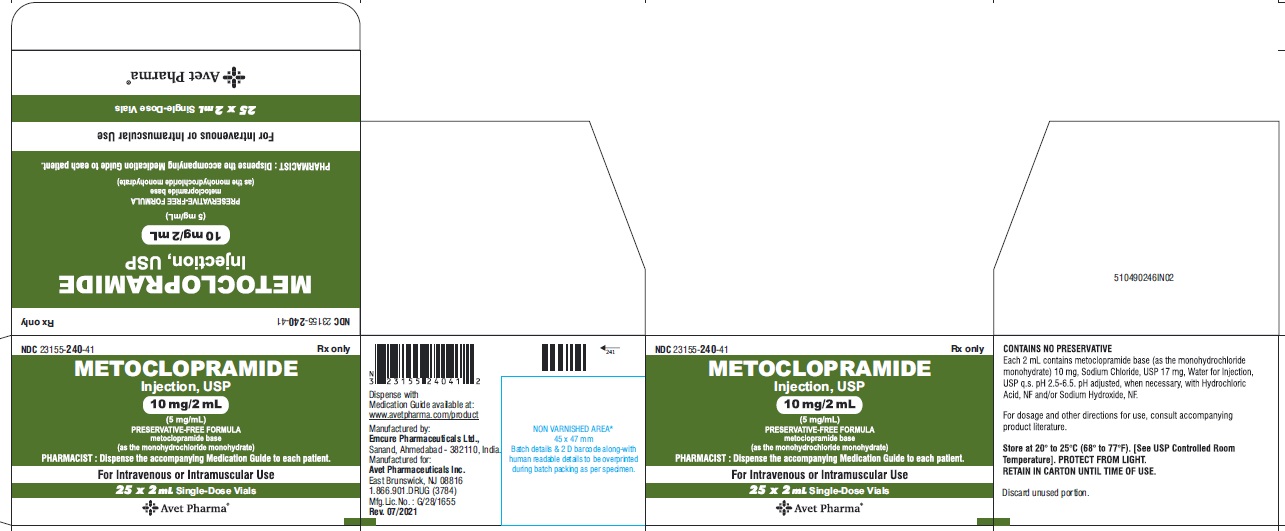
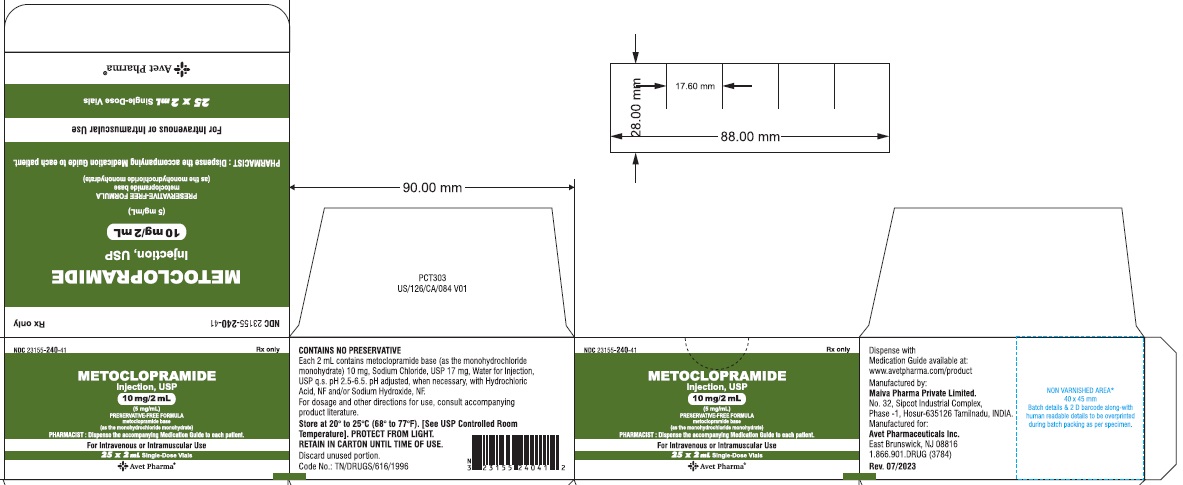
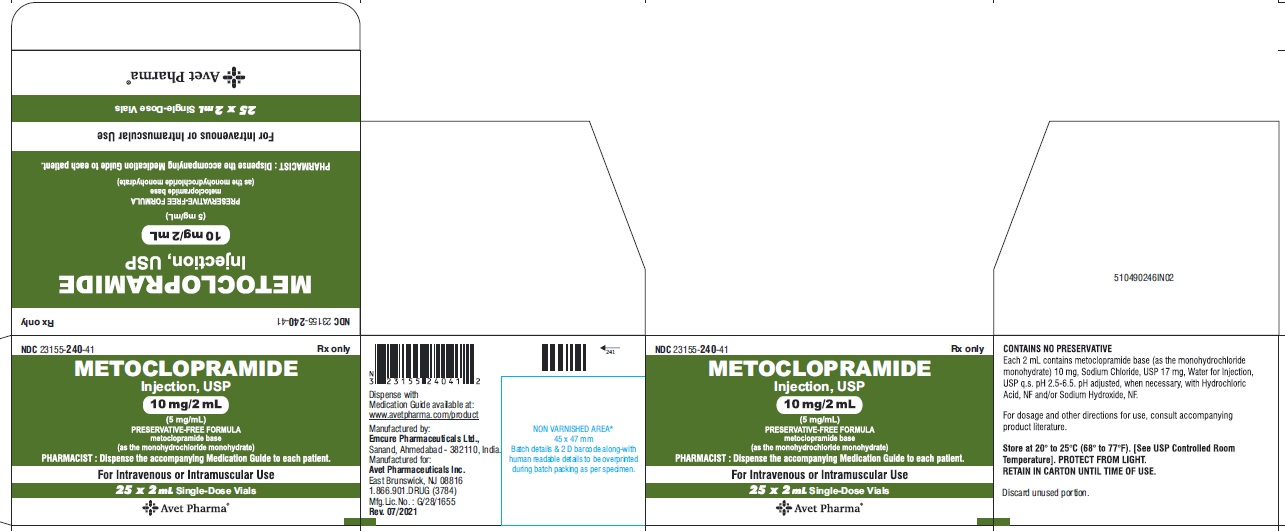
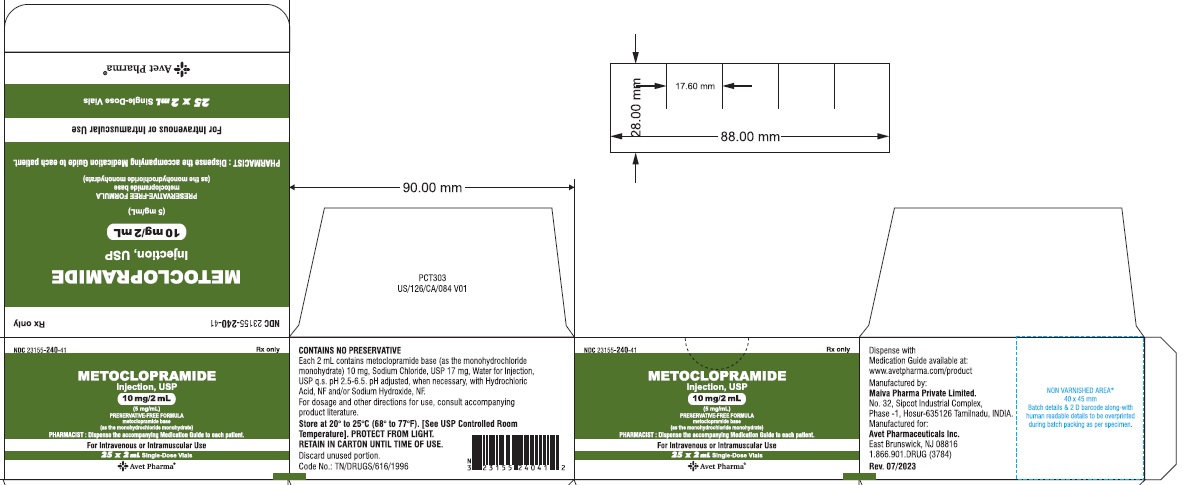
Each 1 mL contains: Metoclopramide base 5 mg (as the monohydrochloride monohydrate), Sodium Chloride, USP 8.5 mg, Water for Injection, USP q.s. pH adjusted, when necessary, with hydrochloric acid and/or sodium hydroxide.
-
CLINICAL PHARMACOLOGY
Metoclopramide stimulates motility of the upper gastrointestinal tract without stimulating gastric, biliary, or pancreatic secretions. Its mode of action is unclear. It seems to sensitize tissues to the action of acetylcholine. The effect of metoclopramide on motility is not dependent on intact vagal innervation, but it can be abolished by anticholinergic drugs.
Metoclopramide increases the tone and amplitude of gastric (especially antral) contractions, relaxes the pyloric sphincter and the duodenal bulb, and increases peristalsis of the duodenum and jejunum resulting in accelerated gastric emptying and intestinal transit. It increases the resting tone of the lower esophageal sphincter. It has little, if any, effect on the motility of the colon or gallbladder.
In patients with gastroesophageal reflux and low LESP (lower esophageal sphincter pressure), single oral doses of metoclopramide produce dose-related increases in LESP. Effects begin at about 5 mg and increase through 20 mg (the largest dose tested). The increase in LESP from a 5 mg dose lasts about 45 minutes and that of 20 mg lasts between 2 and 3 hours. Increased rate of stomach emptying has been observed with single oral doses of 10 mg.
The antiemetic properties of metoclopramide appear to be a result of its antagonism of central and peripheral dopamine receptors. Dopamine produces nausea and vomiting by stimulation of the medullary chemoreceptor trigger zone (CTZ), and metoclopramide blocks stimulation of the CTZ by agents like l-dopa or apomorphine which are known to increase dopamine levels or to possess dopamine-like effects.
Metoclopramide also abolishes the slowing of gastric emptying caused by apomorphine.
Like the phenothiazines and related drugs, which are also dopamine antagonists, metoclopramide produces sedation and may produce extrapyramidal reactions, although these are comparatively rare (see WARNINGS). Metoclopramide inhibits the central and peripheral effects of apomorphine, induces release of prolactin and causes a transient increase in circulating aldosterone levels, which may be associated with transient fluid retention.
The onset of pharmacological action of metoclopramide is 1 to 3 minutes following an intravenous dose, 10 to 15 minutes following intramuscular administration, and 30 to 60 minutes following an oral dose; pharmacological effects persist for 1 to 2 hours.
Pharmacokinetics
Metoclopramide is rapidly and well absorbed. Relative to an intravenous dose of 20 mg, the absolute oral bioavailability of metoclopramide is 80% ± 15.5% as demonstrated in a crossover study of 18 subjects.
Peak plasma concentrations occur at about 1 to 2 hr after a single oral dose. Similar time to peak is observed after individual doses at steady state.
In a single dose study of 12 subjects, the area under the drug concentration-time curve increases linearly with doses from 20 to 100 mg. Peak concentrations increase linearly with dose; time to peak concentrations remains the same; whole body clearance is unchanged; and the elimination rate remains the same. The average elimination half-life in individuals with normal renal function is 5 to 6 hr. Linear kinetic processes adequately describe the absorption and elimination of metoclopramide.
Approximately 85% of the radioactivity of an orally administered dose appears in the urine within 72 hr. Of the 85% eliminated in the urine, about half is present as free or conjugated metoclopramide.
The drug is not extensively bound to plasma proteins (about 30%). The whole body volume of distribution is high (about 3.5 L/kg) which suggests extensive distribution of drug to the tissues.
Renal impairment affects the clearance of metoclopramide. In a study with patients with varying degrees of renal impairment, a reduction in creatinine clearance was correlated with a reduction in plasma clearance, renal clearance, non-renal clearance, and increase in elimination half-life. The kinetics of metoclopramide in the presence of renal impairment remained linear however. The reduction in clearance as a result of renal impairment suggests that adjustment downward of maintenance dosage should be done to avoid drug accumulation.
Adult Pharmacokinetic Data
Parameter
Value
Vd (L/kg)
~ 3.5
Plasma Protein Binding
~ 30%
t1/2 (hr)
5-6
Oral Bioavailability
80%±15.5%
In pediatric patients, the pharmacodynamics of metoclopramide following oral and intravenous administration are highly variable and a concentration-effect relationship has not been established.
There are insufficient reliable data to conclude whether the pharmacokinetics of metoclopramide in adults and the pediatric population are similar. Although there are insufficient data to support the efficacy of metoclopramide in pediatric patients with symptomatic gastroesophageal reflux (GER) or cancer chemotherapy-related nausea and vomiting, its pharmacokinetics have been studied in these patient populations.
In an open-label study, six pediatric patients (age range, 3.5 weeks to 5.4 months) with GER received a metoclopramide 0.15 mg/kg oral solution every 6 hours for 10 doses. The mean peak plasma concentration of metoclopramide after the tenth dose was 2-fold (56.8 mcg/L) higher compared to that observed after the first dose (29 mcg/L) indicating drug accumulation with repeated dosing. After the tenth dose, the mean time to reach peak concentrations (2.2 hr), half-life (4.1 hr), clearance (0.67 L/h/kg), and volume of distribution (4.4 L/kg) of metoclopramide were similar to those observed after the first dose. In the youngest patient (age, 3.5 weeks), metoclopramide half-life after the first and the tenth dose (23.1 and 10.3 hr, respectively) was significantly longer compared to other infants due to reduced clearance. This may be attributed to immature hepatic and renal systems at birth.
Single intravenous doses of metoclopramide 0.22 to 0.46 mg/kg (mean, 0.35 mg/kg) were administered over 5 minutes to 9 pediatric cancer patients receiving chemotherapy (mean age, 11.7 years; range, 7 to 14 yr) for prophylaxis of cytotoxic-induced vomiting. The metoclopramide plasma concentrations extrapolated to time zero ranged from 65 to 395 mcg/L (mean, 152 mcg/L). The mean elimination half-life, clearance, and volume of distribution of metoclopramide were 4.4 hr (range, 1.7 to 8.3 hr), 0.56 L/h/kg (range, 0.12 to 1.20 L/h/kg), and 3.0 L/kg (range, 1.0 to 4.8 L/kg), respectively.
In another study, nine pediatric cancer patients (age range, 1 to 9 yr) received 4 to 5 intravenous infusions (over 30 minutes) of metoclopramide at a dose of 2 mg/kg to control emesis. After the last dose, the peak serum concentrations of metoclopramide ranged from 1060 to 5680 mcg/L. The mean elimination half-life, clearance, and volume of distribution of metoclopramide were 4.5 hr (range, 2.0 to 12.5 hr), 0.37 L/h/kg (range, 0.10 to 1.24 L/h/kg), and 1.93 L/kg (range, 0.95 to 5.50 L/kg), respectively.
Pediatric Pharmacokinetic Studies
Reference
Dose, Route
t1/2
(hr)
Cl
(L/hr/kg)
Vd
(L/kg)
Cmax
(mcg/L)
1.
0.35 mg/kg, IV over 5 min
4.4±0.56
0.56±0.10
3.0±0.38 (Dose/Cp0)
152±31
2.
2 mg/kg
30 min IV
infusion 4-5 times within 9.5 hours
4.5a
0.37a
1.93a
1060 to 5680a
a. SEM not available.
- Bateman, DN, et al. Br J Clin Pharmac 15:557-559, 1983.
- Ford, C. Clin Pharmac Ther 43:196, 1988.
-
INDICATIONS AND USAGE
Diabetic Gastroparesis (Diabetic Gastric Stasis)
Metoclopramide injection, USP (metoclopramide hydrochloride, USP) is indicated for the relief of symptoms associated with acute and recurrent diabetic gastric stasis.
The Prevention of Nausea and Vomiting Associated with Emetogenic Cancer Chemotherapy
Metoclopramide injection, USP is indicated for the prophylaxis of vomiting associated with emetogenic cancer chemotherapy.
The Prevention of Postoperative Nausea and Vomiting
Metoclopramide injection, USP is indicated for the prophylaxis of postoperative nausea and vomiting in those circumstances where nasogastric suction is undesirable.
Small Bowel Intubation
Metoclopramide injection, USP may be used to facilitate small bowel intubation in adults and pediatric patients in whom the tube does not pass the pylorus with conventional maneuvers.
Radiological Examination
Metoclopramide injection, USP may be used to stimulate gastric emptying and intestinal transit of barium in cases where delayed emptying interferes with radiological examination of the stomach and/or small intestine.
-
CONTRAINDICATIONS
Metoclopramide should not be used whenever stimulation of gastrointestinal motility might be dangerous, e.g., in the presence of gastrointestinal hemorrhage, mechanical obstruction, or perforation.
Metoclopramide is contraindicated in patients with pheochromocytoma because the drug may cause a hypertensive crisis, probably due to release of catecholamines from the tumor. Such hypertensive crises may be controlled by phentolamine.
Metoclopramide is contraindicated in patients with known sensitivity or intolerance to the drug.
Metoclopramide should not be used in epileptics or patients receiving other drugs which are likely to cause extrapyramidal reactions, since the frequency and severity of seizures or extrapyramidal reactions may be increased.
-
WARNINGS
Neuroleptic Malignant Syndrome (NMS)
There have been rare reports of an uncommon but potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) associated with metoclopramide. Clinical manifestations of NMS include hyperthermia, muscle rigidity, altered consciousness, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis and cardiac arrhythmias).
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, malignant hyperthermia, drug fever and primary central nervous system (CNS) pathology.
The management of NMS should include 1) immediate discontinuation of metoclopramide and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems for which specific treatments are available. Bromocriptine and dantrolene sodium have been used in treatment of NMS, but their effectiveness have not been established (see ADVERSE REACTIONS).
Extrapyramidal Symptoms (EPS)
Acute Dystonic Reactions
Acute dystonic reactions occur in approximately 1 in 500 patients treated with the usual adult dosages of 30 to 40 mg/day of metoclopramide. These usually are seen during the first 24 to 48 hours of treatment with metoclopramide, occur more frequently in pediatric patients and adult patients less than 30 years of age and are even more frequent at the higher doses used in prophylaxis of vomiting due to cancer chemotherapy. These symptoms may include involuntary movements of limbs and facial grimacing, torticollis, oculogyric crisis, rhythmic protrusion of tongue, bulbar type of speech, trismus, or dystonic reactions resembling tetanus. Rarely, dystonic reactions may present as stridor and dyspnea, possibly due to laryngospasm. If these symptoms should occur, inject 50 mg Benadryl®(diphenhydramine hydrochloride) intramuscularly, and they usually will subside. Cogentin®(benztropine mesylate), 1 to 2 mg intramuscularly, may also be used to reverse these reactions.
Tardive Dyskinesia (see Boxed Warnings)
Treatment with metoclopramide can cause tardive dyskinesia (TD), a potentially irreversible and disfiguring disorder characterized by involuntary movements of the face, tongue, or extremities. The risk of developing tardive dyskinesia increases with the duration of treatment and the total cumulative dose. An analysis of utilization patterns showed that about 20% of patients who used metoclopramide took it for longer than 12 weeks. Treatment with metoclopramide for longer than the recommended 12 weeks should be avoided in all but rare cases where therapeutic benefit is thought to outweigh the risk of developing TD.
Although the risk of developing TD in the general population may be increased among the elderly, women, and diabetics, it is not possible to predict which patients will develop metoclopramide-induced TD. Both the risk of developing TD and the likelihood that TD will become irreversible increase with duration of treatment and total cumulative dose.
Metoclopramide should be discontinued in patients who develop signs or symptoms of TD. There is no known effective treatment for established cases of TD, although in some patients, TD may remit, partially or completely, within several weeks to months after metoclopramide is withdrawn.
Metoclopramide itself may suppress, or partially suppress, the signs of TD, thereby masking the underlying disease process. The effect of this symptomatic suppression upon the long-term course of TD is unknown. Therefore, metoclopramide should not be used for the symptomatic control of TD.
Parkinsonian-like Symptoms
Parkinsonian-like symptoms, including bradykinesia, tremor, cogwheel rigidity, or mask-like facies, have occurred more commonly within the first 6 months after beginning treatment with metoclopramide, but occasionally after longer periods. These symptoms generally subside within 2 to 3 months following discontinuance of metoclopramide. Patients with preexisting Parkinson's disease should be given metoclopramide cautiously, if at all, since such patients may experience exacerbation of parkinsonian symptoms when taking metoclopramide.
Depression
Mental depression has occurred in patients with and without prior history of depression. Symptoms have ranged from mild to severe and have included suicidal ideation and suicide. Metoclopramide should be given to patients with a prior history of depression only if the expected benefits outweigh the potential risks.
-
PRECAUTIONS
General Precautions
In one study in hypertensive patients, intravenously administered metoclopramide was shown to release catecholamines; hence, caution should be exercised when metoclopramide is used in patients with hypertension.
Intravenous injections of undiluted metoclopramide should be made slowly allowing 1 to 2 minutes for 10 mg since a transient but intense feeling of anxiety and restlessness, followed by drowsiness, may occur with rapid administration.
Because metoclopramide produces a transient increase in plasma aldosterone, certain patients, especially those with cirrhosis or congestive heart failure, may be at risk of developing fluid retention and volume overload. If these side effects occur at any time during metoclopramide therapy, the drug should be discontinued.
Intravenous administration of metoclopramide injection diluted in a parenteral solution should be made slowly over a period of not less than 15 minutes.
Giving a promotility drug such as metoclopramide theoretically could put increased pressure on suture lines following a gut anastomosis or closure. This possibility should be considered and weighed when deciding whether to use metoclopramide or nasogastric suction in the prevention of postoperative nausea and vomiting.
Information For Patients
A patient Medication Guide is available for metoclopramide injection. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. Refer to accompanying Medication Guide.
Metoclopramide may impair the mental and/or physical abilities required for the performance of hazardous tasks such as operating machinery or driving a motor vehicle. The ambulatory patient should be cautioned accordingly.
Drug Interactions
The effects of metoclopramide on gastrointestinal motility are antagonized by anticholinergic drugs and narcotic analgesics. Additive sedative effects can occur when metoclopramide is given with alcohol, sedatives, hypnotics, narcotics, or tranquilizers.
The finding that metoclopramide releases catecholamines in patients with essential hypertension suggests that it should be used cautiously, if at all, in patients receiving monoamine oxidase inhibitors.
Absorption of drugs from the stomach may be diminished (e.g., digoxin) by metoclopramide, whereas the rate and/or extent of absorption of drugs from the small bowel may be increased (e.g., acetaminophen, tetracycline, levodopa, ethanol, cyclosporine).
Gastroparesis (gastric stasis) may be responsible for poor diabetic control in some patients. Exogenously administered insulin may begin to act before food has left the stomach and lead to hypoglycemia. Because the action of metoclopramide will influence the delivery of food to the intestines and thus the rate of absorption, insulin dosage or timing of dosage may require adjustment.
Carcinogenesis, Mutagenesis, Impairment of Fertility
A 77-week study was conducted in rats with oral doses up to about 40 times the maximum recommended human daily dose. Metoclopramide elevates prolactin levels and the elevation persists during chronic administration. Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin-dependent in vitro, a factor of potential importance if the prescription of metoclopramide is contemplated in a patient with previously detected breast cancer. Although disturbances such as galactorrhea, amenorrhea, gynecomastia, and impotence have been reported with prolactin-elevating drugs, the clinical significance of elevated serum prolactin levels is unknown for most patients. An increase in mammary neoplasms has been found in rodents after chronic administration of prolactin-stimulating neuroleptic drugs and metoclopramide. Neither clinical studies nor epidemiologic studies conducted to date, however, have shown an association between chronic administration of these drugs and mammary tumorigenesis; the available evidence is too limited to be conclusive at this time.
An Ames mutagenicity test performed on metoclopramide was negative.
Pregnancy Category B
Reproduction studies performed in rats, mice and rabbits by the IM, IV, subcutaneous (SC), and oral routes at maximum levels ranging from 12 to 250 times the human dose have demonstrated no impairment of fertility or significant harm to the fetus due to metoclopramide. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Metoclopramide is excreted in human milk. Caution should be exercised when metoclopramide is administered to a nursing mother.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established except as stated to facilitate small bowel intubation (see OVERDOSAGEand DOSAGE AND ADMINISTRATION).
Care should be exercised in administering metoclopramide to neonates since prolonged clearance may produce excessive serum concentrations (see CLINICAL PHARMACOLOGY — Pharmacokinetics). In addition, neonates have reduced levels of NADH-cytochrome b5 reductase which, in combination with the aforementioned pharmacokinetic factors, make neonates more susceptible to methemoglobinemia (seeOVERDOSAGE).
The safety profile of metoclopramide in adults cannot be extrapolated to pediatric patients. Dystonias and other extrapyramidal reactions associated with metoclopramide are more common in the pediatric population than in adults. (See WARNINGSand ADVERSE REACTIONS — Extrapyramidal Reactions.)
Geriatric Use
Clinical studies of metoclopramide injection did not include sufficient numbers of subjects aged 65 and over to determine whether elderly subjects respond differently from younger subjects.
The risk of developing parkinsonian-like side effects increases with ascending dose. Geriatric patients should receive the lowest dose of metoclopramide injection that is effective. If parkinsonian-like symptoms develop in a geriatric patient receiving metoclopramide injection, metoclopramide injection should generally be discontinued before initiating any specific anti-parkinsonian agents (see WARNINGS).
The elderly may be at greater risk for tardive dyskinesia (see WARNINGS – Tardive Dyskinesia).
Sedation has been reported in metoclopramide injection users. Sedation may cause confusion and manifest as over-sedation in elderly (see CLINICAL PHARMACOLOGY, PRECAUTIONS – Information for Patients
and ADVERSE REACTIONS – CNS Effects).
Metoclopramide injection is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function (see DOSAGE AND ADMINISTRATION - Use in Patients With Renal or Hepatic Impairment).
For these reasons, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased renal function, concomitant disease, or other drug therapy in the elderly (see Use in Patients With Renal or Hepatic Impairment).
Other Special Populations
Patients with NADH-cytochrome b5 reductase deficiency are at an increased risk of developing methemoglobinemia and/or sulfhemoglobinemia when metoclopramide is administered. In patients with G6PD deficiency who experience metoclopramide-induced methemoglobinemia, methylene blue treatment is not recommended (see OVERDOSAGE).
-
ADVERSE REACTIONS
In general, the incidence of adverse reactions correlates with the dose and duration of metoclopramide administration. The following reactions have been reported, although in most instances, data do not permit an estimate of frequency:
CNS Effects
Restlessness, drowsiness, fatigue, and lassitude may occur in patients receiving the recommended prescribed dosage of metoclopramide injection. Insomnia, headache, confusion, dizziness, or mental depression with suicidal ideation also may occur (see WARNINGS). In cancer chemotherapy patients being treated with 1 to 2 mg/kg per dose, incidence of drowsiness is about 70%. There are isolated reports of convulsive seizures without clear-cut relationship to metoclopramide. Rarely, hallucinations have been reported.
Extrapyramidal Reactions (EPS)
Acute dystonic reactions, the most common type of EPS associated with metoclopramide, occur in approximately 0.2% of patients (1 in 500) treated with 30 to 40 mg of metoclopramide per day. In cancer chemotherapy patients receiving 1 to 2 mg/kg per dose, the incidence is 2% in patients over the ages of 30 to 35, and 25% or higher in pediatric patients and adult patients less than 30 years of age who have not had prophylactic administration of diphenhydramine. Symptoms include involuntary movements of limbs, facial grimacing, torticollis, oculogyric crisis, rhythmic protrusion of tongue, bulbar type of speech, trismus, opisthotonus (tetanus-like reactions), and, rarely, stridor and dyspnea possibly due to laryngospasm; ordinarily these symptoms are readily reversed by diphenhydramine (see WARNINGS).
Parkinsonian-like symptoms may include bradykinesia, tremor, cogwheel rigidity, mask-like facies (see WARNINGS).
Tardive dyskinesia most frequently is characterized by involuntary movements of the tongue, face, mouth, or jaw, and sometimes by involuntary movements of the trunk and/or extremities; movements may be choreoathetotic in appearance (see WARNINGS).
Motor restlessness (akathisia) may consist of feelings of anxiety, agitation, jitteriness, and insomnia, as well as inability to sit still, pacing, foot tapping. These symptoms may disappear spontaneously or respond to a reduction in dosage.
Neuroleptic Malignant Syndrome
Rare occurrences of neuroleptic malignant syndrome (NMS) have been reported. This potentially fatal syndrome is comprised of the symptom complex of hyperthermia, muscular rigidity, altered consciousness, and autonomic instability (see WARNINGS).
Endocrine Disturbances
Galactorrhea, amenorrhea, gynecomastia, impotence secondary to hyperprolactinemia (see PRECAUTIONS). Fluid retention secondary to transient elevation of aldosterone (see CLINICAL PHARMACOLOGY).
Cardiovascular
Hypotension, hypertension, supraventricular tachycardia, bradycardia, fluid retention, acute congestive heart failure and possible atrioventricular (AV) block (see CONTRAINDICATIONS and PRECAUTIONS).
Gastrointestinal
Nausea and bowel disturbances, primarily diarrhea.
Hepatic
Rarely, cases of hepatotoxicity, characterized by such findings as jaundice and altered liver function tests, when metoclopramide was administered with other drugs with known hepatotoxic potential.
Renal
Urinary frequency and incontinence.
Hematologic
A few cases of neutropenia, leukopenia, or agranulocytosis, generally without clear-cut relationship to metoclopramide. Methemoglobinemia in adults and especially with overdosage in neonates (see OVERDOSAGE). Sulfhemoglobinemia in adults.
Allergic Reactions
A few cases of rash, urticaria, or bronchospasm, especially in patients with a history of asthma. Rarely, angioneurotic edema, including glossal or laryngeal edema.
Miscellaneous
Visual disturbances. Porphyria.
Transient flushing of the face and upper body, without alterations in vital signs, following high doses intravenously.
-
OVERDOSAGE
Symptoms of overdosage may include drowsiness, disorientation and extrapyramidal reactions. Anticholinergic or antiparkinson drugs or antihistamines with anticholinergic properties may be helpful in controlling the extrapyramidal reactions. Symptoms are self-limiting and usually disappear within 24 hours.
Hemodialysis removes relatively little metoclopramide, probably because of the small amount of the drug in blood relative to tissues. Similarly, continuous ambulatory peritoneal dialysis does not remove significant amounts of drug. It is unlikely that dosage would need to be adjusted to compensate for losses through dialysis. Dialysis is not likely to be an effective method of drug removal in overdose situations.
Unintentional overdose due to misadministration has been reported in infants and children with the use of metoclopramide syrup. While there was no consistent pattern to the reports associated with these overdoses, events included seizures, extrapyramidal reactions, and lethargy.
Methemoglobinemia has occurred in premature and full-term neonates who were given overdoses of metoclopramide (1 to 4 mg/kg/day orally, intramuscularly or intravenously for 1 to 3 or more days). Methemoglobinemia can be reversed by the intravenous administration of methylene blue. However, methylene blue may cause hemolytic anemia in patients with G6PD deficiency, which may be fatal (see PRECAUTIONS – Other Special Populations).
-
DOSAGE AND ADMINISTRATION
For the Relief of Symptoms Associated with Diabetic Gastroparesis (Diabetic Gastric Stasis)
If only the earliest manifestations of diabetic gastric stasis are present, oral administration of metoclopramide may be initiated. However, if severe symptoms are present, therapy should begin with metoclopramide injection (IM or IV). Doses of 10 mg may be administered slowly by the intravenous route over a 1- to 2-minute period.
Administration of metoclopramide injection up to 10 days may be required before symptoms subside, at which time oral administration of metoclopramide may be instituted. The physician should make a thorough assessment of the risks and benefits prior to prescribing further metoclopramide treatment.
For the Prevention of Nausea and Vomiting Associated with Emetogenic Cancer Chemotherapy
Intravenous infusions should be made slowly over a period of not less than 15 minutes, 30 minutes before beginning cancer chemotherapy and repeated every 2 hours for two doses, then every 3 hours for three doses.
The initial two doses should be 2 mg/kg if highly emetogenic drugs such as cisplatin or dacarbazine are used alone or in combination. For less emetogenic regimens, 1 mg/kg per dose may be adequate.
For doses in excess of 10 mg, metoclopramide injection should be diluted in 50 mL of a parenteral solution.
The preferred parenteral solution is Sodium Chloride Injection (normal saline), which when combined with metoclopramide injection, can be stored frozen for up to 4 weeks. Metoclopramide injection is degraded when admixed and frozen with Dextrose-5% in Water. Metoclopramide injection diluted in Sodium Chloride Injection, Dextrose-5% in Water, Dextrose-5% in 0.45% Sodium Chloride, Ringer's Injection, or Lactated Ringer's Injection may be stored up to 48 hours (without freezing) after preparation if protected from light. All dilutions may be stored unprotected from light under normal light conditions up to 24 hours after preparation.
If acute dystonic reactions should occur, inject 50 mg Benadryl®(diphenhydramine hydrochloride) intramuscularly, and the symptoms usually will subside.
For the Prevention of Postoperative Nausea and Vomiting
Metoclopramide injection should be given intramuscularly near the end of surgery. The usual adult dose is 10 mg; however, doses of 20 mg may be used.
To Facilitate Small Bowel Intubation
If the tube has not passed the pylorus with conventional maneuvers in 10 minutes, a single dose (undiluted) may be administered slowly by the intravenous route over a 1- to 2-minute period.
The recommended single dose is: Pediatric patients above 14 years of age and adults — 10 mg metoclopramide base. Pediatric patients (6 to 14 years of age) — 2.5 to 5 mg metoclopramide base; (under 6 years of age) — 0.1 mg/kg metoclopramide base.
To Aid in Radiological Examinations
In patients where delayed gastric emptying interferes with radiological examination of the stomach and/or small intestine, a single dose may be administered slowly by the intravenous route over a 1- to 2-minute period.
For dosage, see intubation above.
Use in Patients With Renal or Hepatic Impairment
Since metoclopramide is excreted principally through the kidneys, in those patients whose creatinine clearance is below 40 mL/min, therapy should be initiated at approximately one-half the recommended dosage. Depending upon clinical efficacy and safety considerations, the dosage may be increased or decreased as appropriate.
See OVERDOSAGEsection for information regarding dialysis.
Metoclopramide undergoes minimal hepatic metabolism, except for simple conjugation. Its safe use has been described in patients with advanced liver disease whose renal function was normal.
NOTE: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
ADMIXTURES COMPATIBILITIES
Metoclopramide injection is compatible for mixing and injection with the following dosage forms to the extent indicated below:
Physically and Chemically Compatible Up to 48 Hours
Cimetidine Hydrochloride (SK&F), Mannitol, USP (Abbott), Potassium Acetate, USP (Invenex), Potassium Phosphate, USP (Invenex).
Physically Compatible Up to 48 Hours
Ascorbic Acid, USP (Abbott), Benztropine Mesylate, USP (MS&D), Cytarabine, USP (Upjohn), Dexamethasone Sodium Phosphate, USP (ESI, MS&D), Diphenhydramine Hydrochloride, USP (Parke-Davis), Doxorubicin Hydrochloride, USP (Adria), Heparin Sodium, USP (ESI), Hydrocortisone Sodium Phosphate (MS&D), Lidocaine Hydrochloride, USP (ESI), Multi-Vitamin Infusion (must be refrigerated-USV), Vitamin B Complex with Ascorbic Acid (Roche).
Physically Compatible Up to 24 Hours
(Do not use if precipitation occurs)
Clindamycin Phosphate, USP (Upjohn), Cyclophosphamide, USP (Mead-Johnson), Insulin, USP (Lilly).
Conditionally Compatible
(Use within one hour after mixing or may be infused directly into the same running IV line)
Ampicillin Sodium, USP (Bristol), Cisplatin (Bristol), Erythromycin Lactobionate, USP (Abbott), Methotrexate Sodium, USP (Lederle), Penicillin G Potassium, USP (Squibb), Tetracycline Hydrochloride, USP (Lederle).
Incompatible
(Do Not Mix)
Cephalothin Sodium, USP (Lilly), Chloramphenicol Sodium, USP (Parke-Davis), Sodium Bicarbonate, USP (Abbott).
-
HOW SUPPLIED
Metoclopramide Injection, USP 5 mg metoclopramide base (as the monohydrochloride monohydrate) per mL; available in:
2 mL single dose vials (NDC 23155-240-31 ) in cartons of 25 (NDC 23155-240-41),
10 mL single dose vials (NDC 23155-240-32) in cartons of 25 (NDC 23155-240-42),
30 mL single dose vials (NDC 23155-240-33) in cartons of 25 (NDC 23155-240-43).
Container Total Contents # Concentration # Administration 2 mL single dose vial 10 mg 5 mg/mL FOR IV or IM ADMINISTRATION 10 mL single dose vial 50 mg 5 mg/mL FOR IV INFUSION ONLY;
DILUTE BEFORE USING30 mL single dose vial 150 mg 5 mg/mL FOR IV INFUSION ONLY;
DILUTE BEFORE USING# Metoclopramide base (as the monohydrochloride monohydrate) Retain in carton until time of use. Do not store open single dose vials for later use, as they contain no preservative.
This product is light sensitive. It should be inspected before use and discarded if either color or particulate is observed.
Dilutions may be stored unprotected from light under normal light conditions up to 24 hours after preparation.
Metoclopramide Injection, USP should be stored at 20° to 25°C (68° to 77°F), excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Other brands mentioned are trademarks of their respective owners.
Dispense with Medication Guide available at: www.avetpharma.com/product
Manufactured by:
Emcure Pharmaceuticals Ltd.,
Sanand, Ahmedabad - 382110, India.
Manufactured for:
Avet Pharmaceuticals Inc.
East Brunswick, NJ 08816
1.866.901.DRUG (3784)

Revised: 08/2021
OR
Manufactured by:
Maiva Pharma Private Limited.
No. 32, Sipcot Industrial Complex,
Phase -1, Hosur-635126 Tamilnadu, INDIA.
Manufactured for:Avet Pharmaceuticals Inc.
East Brunswick, NJ 08816
1.866.901.DRUG (3784)

Revised: 02/2024
-
MEDICATION GUIDE
Metoclopramide (met" oh kloe' pra mide) Injection, USP
You or your caregiver should read the Medication Guide before you start receiving metoclopramide injection, USP and before you get another dose of metoclopramide injection, USP. There may be new information. If you take another product that contains metoclopramide, you should read the Medication Guide that comes with that product. Some of the information may be different. This Medication Guide does not take the place of talking to your doctor about your medical condition or your treatment.
What is the most important information I should know about metoclopramide injection, USP?
Metoclopramide injection, USP can cause serious side effects, including:
Abnormal muscle movements called tardive dyskinesia (TD). These movements happen mostly in the face muscles. You can not control these movements. They may not go away even after stopping metoclopramide injection, USP. There is no treatment for TD, but symptoms may lessen or go away over time after you stop taking metoclopramide injection, USP.
Your chances for getting TD go up:
- the longer you take metoclopramide injection, USP and the more metoclopramide injection, USP you take. You should not take metoclopramide injection, USP for more than 12 weeks.
- if you are older, especially if you are a woman
- if you have diabetes
It is not possible for your doctor to know if you will get TD if you take metoclopramide injection, USP.
Call your doctor right away if you get movements you can not stop or control, such as:
- lip smacking, chewing, or puckering up your mouth
- frowning or scowling
- sticking out your tongue
- blinking and moving your eyes
- shaking of your arms and legs
See the section "What are the possible side effects of metoclopramide injection, USP?" for more information about side effects.
What is metoclopramide injection, USP?
Metoclopramide injection, USP is a prescription medicine used to:
- relieve symptoms of slow stomach emptying in people with diabetes
- prevent nausea and vomiting that can happen with cancer chemotherapy
- prevent nausea and vomiting that may happen after surgery, if your doctor decides that you should not be treated with a stomach tube and suction
- help make it easier to insert a tube into the small intestine in both adults and children, if the tube does not pass into the stomach normally.
- to help empty stomach contents or to help barium move through your intestine, when you get an X-ray examination of the stomach or small intestine. It is not known if metoclopramide injection, USP is safe and works in children except when used to help insert a tube into the small intestine.
Who should not receive metoclopramide injection, USP?
Do not receive metoclopramide injection, USP if you:
- have stomach or intestine problems that could get worse with metoclopramide injection, USP, such as bleeding, blockage or a tear in your stomach or bowel wall
- have an adrenal gland tumor called pheochromocytoma
- are allergic to metoclopramide injection, USP or anything in it. See the end of this Medication Guide for a list of ingredients in metoclopramide injection, USP.
- take medicines that can cause uncontrolled movements, such as medicines for mental illness
- have seizures
What should I tell my doctor before receiving metoclopramide injection, USP?
Tell your doctor about all of your medical conditions,
including if you have:
- depression
- Parkinson's disease
- high blood pressure
- kidney problems. Your doctor may start with a lower dose.
- liver problems or heart failure. Metoclopramide injection, USP may cause your body to hold fluids.
- diabetes. Your dose of insulin may need to be changed.
- breast cancer
- you are pregnant or plan to become pregnant. It is not known if metoclopramide injection, USP will harm your unborn child.
- you are breastfeeding. Metoclopramide injection, USP is passed into human milk and may harm your baby. Talk with your doctor about the best way to feed your baby if you take metoclopramide injection, USP.
Tell your doctor about all the medicines you take, including prescription and non prescription medicines, vitamins and herbal supplements.
Metoclopramide injection, USP and some other medicines can affect each other and may not work as well, or cause possible side effects. Do not start any new medicines while receiving metoclopramide injection, USP until you talk with your doctor.
Especially tell your doctor if you take:
- another medicine that contains metoclopramide.
- a blood pressure medicine
- a medicine for depression, especially a Monoamine Oxidase Inhibitor (MAOI)
- insulin
- a medicine that can make you sleepy, such as anti-anxiety medicine, sleep medicines, and narcotics.
If you are not sure if your medicine is one listed above, ask your doctor or pharmacist. Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
How will I receive metoclopramide injection, USP?
- Metoclopramide injection, USP will be given to you by intravenous (IV) infusion into your vein or by intramuscular (IM) injection into a large muscle. Where and how you receive your metoclopramide injection (IV or IM) will depend on why you are receiving it.
- Certain side effects can happen if metoclopramide injection, USP is given too fast. See the section "What are the possible side effects of metoclopramide injection, USP?"
- You should not take or receive metoclopramide injection, USP for more than 12 weeks.
What should I avoid while receiving metoclopramide injection, USP?
- Do not drink alcohol while receiving metoclopramide injection, USP. Alcohol may make some side effects of metoclopramide injection, USP worse, such as feeling sleepy.
- Do not drive, work with machines, or do dangerous tasks until you know how metoclopramide injection, USP affects you. Metoclopramide injection, USP may cause sleepiness.
What are the possible side effects of metoclopramide injection, USP?
Metoclopramide injection, USP can cause serious side effects, including:
- Abnormal muscle movements. See the section "What is the most important information I should know about metoclopramide injection, USP?"
- Uncontrolled spasms of your face and neck muscles, or muscles of your body, arms, and legs (dystonia). These muscle spasms can cause abnormal movements and body positions. These spasms usually start within the first 2 days of treatment. These spasms happen more often in children and adults under age 30.
- Depression, thoughts about suicide, and suicide. Some people who take metoclopramide injection, USP become depressed. You may have thoughts about hurting or killing yourself. Some people who take metoclopramide injection, USP have ended their own lives (suicide).
- Neuroleptic Malignant Syndrome (NMS). NMS is a very rare but very serious condition that can happen with metoclopramide injection, USP. NMS can cause death and must be treated in a hospital. Symptoms of NMS include: high fever, stiff muscles, problems thinking, very fast or uneven heartbeat, and increased sweating.
- Parkinsonism. Symptoms include slight shaking, body stiffness, trouble moving or keeping your balance. If you already have Parkinson's disease, your symptoms may become worse while you are receiving metoclopramide injection, USP.
Call your doctor and get medical help right away if you:
- feel depressed or have thoughts about hurting or killing yourself
- have high fever, stiff muscles, problems thinking, very fast or uneven heartbeat, and increased sweating
- have muscle movements you can not stop or control
- have muscle movements that are new or unusual
Common side effects of metoclopramide injection, USP include:
- feeling restless, sleepy, tired, dizzy, or exhausted
- headache
- confusion
- trouble sleeping
Infusion related side effects can happen if metoclopramide injection, USP is given too fast. You may feel very anxious and restless for a short time, and then become sleepy while you are receiving a dose of metoclopramide injection, USP. Tell your doctor or nurse right away if this happens.
You may have more side effects the longer you take metoclopramide injection, USP and the more metoclopramide injection, USP you take.
Tell your doctor about any side effects that bother you or do not go away. These are not all the possible side effects of metoclopramide injection, USP.
Call your doctor for medical advice about side effects. You may report side effects to Avet Pharmaceuticals Inc. at 1-866-901-DRUG (3784) or FDA at 1-800-FDA-1088.
General information about metoclopramide injection, USP
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
This Medication Guide summarizes the most important information about metoclopramide injection, USP. If you would like more information about metoclopramide injection, USP, talk with your doctor. You can ask your doctor or pharmacist for information about metoclopramide injection, USP that is written for healthcare professionals. For more information, call Avet Pharmaceuticals Inc. at 1-866-901-DRUG (3784).
What are the ingredients in metoclopramide injection, USP?
Active ingredient: metoclopramide, USP
Inactive ingredients: sodium chloride, water, hydrochloric acid or sodium hydroxide
Dispense with Medication Guide available at: www.avetpharma.com/product
Manufactured by:
Emcure Pharmaceuticals Ltd.,
Sanand, Ahmedabad - 382110, India.
Manufactured for:
Avet Pharmaceuticals Inc.
East Brunswick, NJ 08816
1.866.901.DRUG (3784)

Revised: 08/2021
OR
Manufactured by:
Maiva Pharma Private Limited.
No. 32, Sipcot Industrial Complex,
Phase -1, Hosur-635126 Tamilnadu, INDIA.
Manufactured for:
Avet Pharmaceuticals Inc.
East Brunswick, NJ 08816
1.866.901.DRUG (3784)

Revised: 02/2024
This Medication Guide has been approved by the U.S. Food and Drug Administration.
- Package Label Principal Display Panel - 10 mg/2 mL - Label
- Package Label Principal Display Panel - 10 mg/2 mL - carton
-
INGREDIENTS AND APPEARANCE
METOCLOPRAMIDE
metoclopramide hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:23155-240 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METOCLOPRAMIDE HYDROCHLORIDE (UNII: W1792A2RVD) (METOCLOPRAMIDE - UNII:L4YEB44I46) METOCLOPRAMIDE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23155-240-42 25 in 1 CARTON 01/03/2014 1 NDC:23155-240-32 10 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC:23155-240-41 25 in 1 CARTON 01/03/2014 2 NDC:23155-240-31 2 mL in 1 VIAL; Type 0: Not a Combination Product 3 NDC:23155-240-43 25 in 1 CARTON 01/03/2014 3 NDC:23155-240-33 30 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204756 01/03/2014 Labeler - Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc. (780779901) Registrant - AVET LIFESCIENCES PRIVATE LIMITED (853181664) Establishment Name Address ID/FEI Business Operations Emcure Pharmaceuticals Limited 675467924 ANALYSIS(23155-240) , MANUFACTURE(23155-240) , PACK(23155-240) , LABEL(23155-240) Establishment Name Address ID/FEI Business Operations Maiva Pharma Private Limited 725656438 analysis(23155-240) , manufacture(23155-240) , label(23155-240) , pack(23155-240)