Label: PSEUDOEPHEDRINE HYDROCHLORIDE tablet
- NDC Code(s): 0904-6728-46, 0904-6728-52, 0904-6907-06
- Packager: Major Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- PURPOSE
- Uses
-
WARNINGS
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- diabetes
- heart disease
- high blood pressure
- thyroid disease
- trouble urinating due to an enlarged prostate gland
- Directions
- Other information
- INACTIVE INGREDIENT
- Questions or comments?
- SPL UNCLASSIFIED SECTION
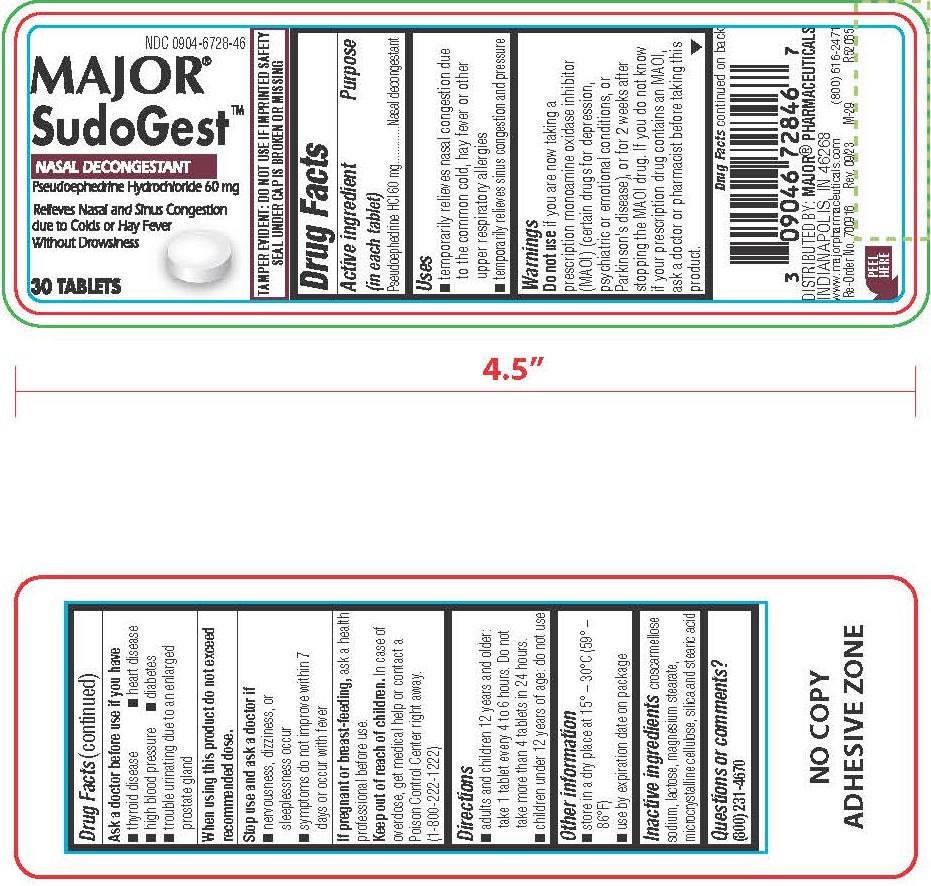
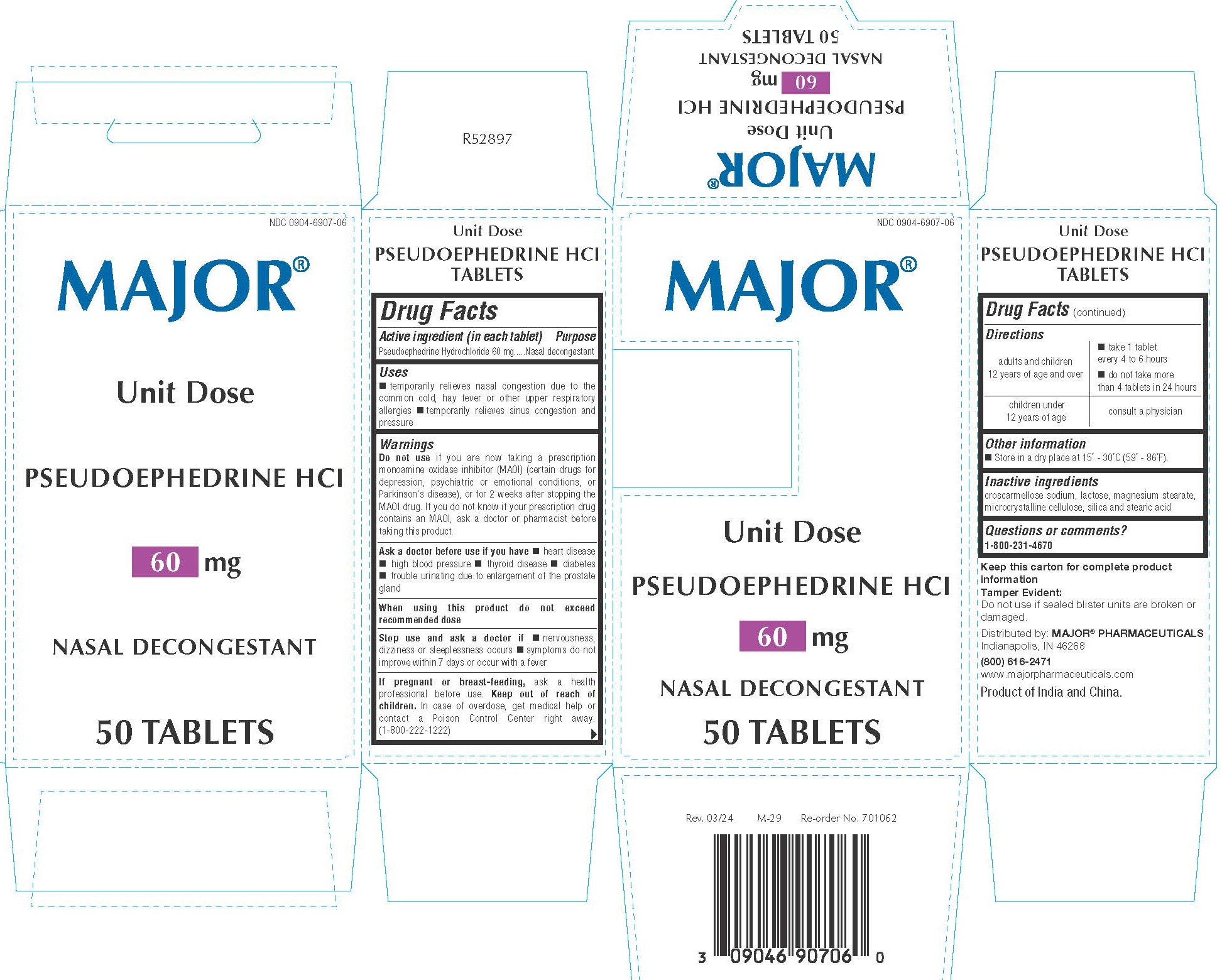
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PSEUDOEPHEDRINE HYDROCHLORIDE
pseudoephedrine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-6728 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white Score no score Shape ROUND Size 8mm Flavor Imprint Code CPC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-6728-52 1 in 1 CARTON 08/26/2019 1 100 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:0904-6728-46 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/29/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 08/26/2019 PSEUDOEPHEDRINE HYDROCHLORIDE
pseudoephedrine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-6907 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white Score no score Shape ROUND Size 8mm Flavor Imprint Code CPC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-6907-06 5 in 1 BOX 04/11/2019 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/31/2019 Labeler - Major Pharmaceuticals (191427277)