Label: LIVTENCITY- maribavir tablet, coated
- NDC Code(s): 64764-800-28, 64764-800-56
- Packager: Takeda Pharmaceuticals America, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 4, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LIVTENCITY safely and effectively. See full prescribing information for LIVTENCITY. LIVTENCITY® (maribavir) tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGELIVTENCITY is indicated for the treatment of adults and pediatric patients (12 years of age and older and weighing at least 35 kg) with post-transplant cytomegalovirus (CMV) infection/disease that ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage in adults and pediatric patients (12 years of age and older and weighing at least 35 kg) is 400 mg (two 200 mg tablets) taken orally twice daily ...
-
3 DOSAGE FORMS AND STRENGTHSTablet: 200 mg, blue, oval shaped convex tablet debossed with "SHP" on one side and "620" on the other side.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Risk of Reduced Antiviral Activity When Coadministered with Ganciclovir and Valganciclovir - LIVTENCITY may antagonize the antiviral activity of ganciclovir and valganciclovir by inhibiting ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Reduced Antiviral Activity When Coadministered with Ganciclovir or Valganciclovir - LIVTENCITY is not recommended to be coadministered with valganciclovir/ganciclovir (vGCV/GCV). LIVTENCITY ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - No adequate human data are available to establish whether LIVTENCITY poses a risk to pregnancy outcomes. In animal reproduction studies, embryo-fetal survival ...
-
10 OVERDOSAGEThere is no known specific antidote for LIVTENCITY. In case of overdose, it is recommended that the patient be monitored for adverse reactions and appropriate symptomatic treatment instituted. Due ...
-
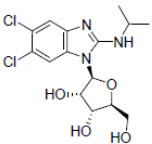
11 DESCRIPTIONLIVTENCITY tablets contain maribavir, a benzimidazole riboside CMV pUL97 protein kinase inhibitor. The chemical name of maribavir is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - LIVTENCITY is an antiviral drug against human CMV [see Microbiology (12.4)]. 12.2 Pharmacodynamics - Exposure-Response - In dose-ranging studies that evaluated ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Two-year carcinogenicity studies were conducted in mice and rats administered oral doses up to 150 and 100 mg/kg/day, respectively ...
-
14 CLINICAL STUDIES14.1 Treatment of Adults with Post-Transplant CMV Infection/Disease That Is Refractory (with or without Genotypic Resistance) to Ganciclovir, Valganciclovir, Cidofovir, or Foscarnet ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTablet: 200 mg, blue, oval shaped convex tablet debossed with "SHP" on one side and "620" on the other side. They are supplied as follows: Bottles of 28 tablets with child-resistant caps (NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Inform patients that LIVTENCITY may interact with other drugs. Advise patients to ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Takeda Pharmaceuticals America, Inc. Lexington, MA 02421 - LIVTENCITY® and the LIVTENCITY Logo® are registered trademarks of Takeda Pharmaceuticals International AG. TAKEDA® and ...
-
PATIENT PACKAGE INSERTPatient Information - LIVTENCITY (liv-TEN-city) (maribavir) tablets - This Patient Information has been approved by the U.S. Food and Drug Administration. MAR358 R4Revised: March ...
-
Instructions for UseLIVTENCITY (liv-TEN-city) (maribavir) tablets, for oral use - This Instructions for Use contains information on how to prepare and give a dose of LIVTENCITY tablets by breaking apart (dispersing ...
-
PRINCIPAL DISPLAY PANEL - 200 mg Bottle CartonNDC 64764-800-28 - LIVTENCITY - (maribavir) tablets - 200 mg - Rx Only - 28 Tablets - For Oral Use - Takeda
-
INGREDIENTS AND APPEARANCEProduct Information