Label: KYBELLA- deoxycholic acid injection, solution
-
NDC Code(s):
61168-101-01,
61168-101-03,
61168-101-04,
61168-101-91, view more61168-101-94
- Packager: Kythera Biopharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use KYBELLA safely and effectively. See full prescribing information for KYBELLA.
KYBELLA® (deoxycholic acid) injection, for subcutaneous use
Initial U.S. Approval: 2015
RECENT MAJOR CHANGES
Warnings and Precautions (5.6) 10/2022 INDICATIONS AND USAGE
KYBELLA is a cytolytic drug indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults. (1)
Limitations of use
The safe and effective use of KYBELLA for the treatment of subcutaneous fat outside the submental region has not been established and is not recommended. (1)
DOSAGE AND ADMINISTRATION
- 0.2 mL injections spaced 1 cm apart until all sites in the planned treatment area have been injected. (2.1)
- Up to 50 injections or 10 mL may be injected in a single treatment. (2.1)
- Up to 6 single treatments may be administered at intervals no less than 1-month apart. (2.1)
- See General Considerations for Administration and Injection Technique before injection. (2.2, 2.3)
DOSAGE FORMS AND STRENGTHS
Injection: 10 mg/mL. (3)
CONTRAINDICATIONS
KYBELLA is contraindicated:
- In the presence of infection at the injection sites. (4)
WARNINGS AND PRECAUTIONS
- Marginal mandibular nerve (MMN) injury: Follow injection technique to avoid this injury. (2.3, 5.1)
- Dysphagia may occur with KYBELLA use. Use in patients with pre-existing dysphagia may exacerbate the condition. (5.2)
- Submental hematoma/bruising occurs frequently after KYBELLA administration. Use with caution in patients who are being treated with antiplatelet or anticoagulant therapy or have coagulation abnormalities. (5.3)
- Avoid injecting in proximity to vulnerable anatomic structures due to the increased risk of tissue damage and vascular injury. (2.3, 5.4)
- Injection site alopecia: Withhold subsequent treatments until resolution. (5.5)
- Injection site ulceration, necrosis, and infection: Do not administer to the affected area until complete resolution. (5.6)
ADVERSE REACTIONS
The most common adverse reactions (>20% of subjects) include injection site edema/swelling, hematoma, pain, numbness, erythema and induration. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2023
- 0.2 mL injections spaced 1 cm apart until all sites in the planned treatment area have been injected. (2.1)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 General Considerations for Administration
2.3 Injection Technique
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Marginal mandibular nerve injury
5.2 Dysphagia
5.3 Injection site hematoma/bruising
5.4 Risk of injecting in proximity to vulnerable anatomic structures
5.5 Injection site alopecia
5.6 Injection site ulceration, necrosis, and infection
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1
INDICATIONS AND USAGE
KYBELLA® (deoxycholic acid) injection is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults.
Limitations of use
The safe and effective use of KYBELLA for the treatment of subcutaneous fat outside the submental region has not been established and is not recommended.
-
2
DOSAGE AND ADMINISTRATION
2.1 Dosage
KYBELLA injection is injected into subcutaneous fat tissue in the submental area using an area-adjusted dose of 2 mg/cm2.
- A single treatment consists of up to a maximum of 50 injections, 0.2 mL each (up to a total of 10 mL), spaced 1 cm apart.
- Up to 6 single treatments may be administered at intervals no less than 1 month apart.
See General Considerations for Administration (2.2) and Injection Technique (2.3) before injection.
2.2 General Considerations for Administration
KYBELLA should be administered by a healthcare professional.
Screen patients for other potential causes of submental convexity/fullness (e.g., thyromegaly and cervical lymphadenopathy).
Give careful consideration to the use of KYBELLA in patients with excessive skin laxity, prominent platysmal bands or other conditions for which reduction of submental fat may result in an aesthetically undesirable outcome.
Use caution in patients who have had prior surgical or aesthetic treatment of the submental area. Changes in anatomy/landmarks or the presence of scar tissue may impact the ability to safely administer KYBELLA or to obtain the desired aesthetic result.
KYBELLA is clear, colorless and free of particulate matter. Visually inspect KYBELLA vials for particulate matter and/or discoloration, and discard the vial if the solution is discolored and/or contains particulate matter.
After use, discard any remaining solution in the vial.
2.3 Injection Technique
The safe and effective use of KYBELLA depends on the use of the correct number and locations for injections, proper needle placement, and administration techniques.
Healthcare professionals administering KYBELLA must understand the relevant submental anatomy and associated neuromuscular structures in the area involved and any alterations to the anatomy due to prior surgical or aesthetic procedures [see Warnings and Precautions (5)].
Avoid injections near the area of the marginal mandibular nerve
Needle placement with respect to the mandible is very important as it reduces the potential for injury to the marginal mandibular nerve, a motor branch of the facial nerve. Injury to the nerve presents as an asymmetrical smile due to paresis of lip depressor muscles [see Warnings and Precautions (5.1)].
To avoid injury to the marginal mandibular nerve:
- Do not inject above the inferior border of the mandible.
- Do not inject within a region defined by a 1-1.5 cm line below the inferior border (from the angle of the mandible to the mentum).
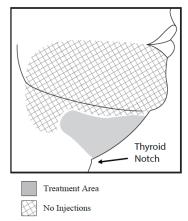
- Inject KYBELLA only within the target submental fat treatment area (see Figures 1 and 3).
Figure 1. Avoid the Marginal Mandibular Nerve Area
Avoid injection into the platysma
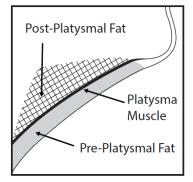
Prior to each treatment session, palpate the submental area to ensure sufficient submental fat and to identify subcutaneous fat between the dermis and platysma (pre-platysmal fat) within the target treatment area (Figure 2). The number of injections and the number of treatments should be tailored to the individual patient’s submental fat distribution and treatment goals.
Figure 2. Sagittal View of Platysma Area
Injecting into the treatment area
Use of ice/cold packs, topical and/or injectable local anesthesia (e.g., lidocaine) may enhance patient comfort.
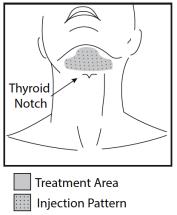
Outline the planned treatment area with a surgical pen and apply a 1 cm injection grid to mark the injection sites (Figures 2 and 3).
Figure 3. Treatment Area and Injection Pattern
Do not inject KYBELLA outside the defined parameters [see Warnings and Precautions (5.1, 5.4, 5.6)].
- Using a large bore needle, draw 1 mL of KYBELLA into a sterile 1 mL syringe and expel any air bubbles in the syringe barrel.
- Have the patient tense the platysma. Pinch the submental fat and, using a 30 gauge (or smaller) 0.5 inch needle, inject 0.2 mL of KYBELLA into the pre-platysmal fat (see Figure 2) next to each of the marked injection sites by advancing the needle perpendicular to the skin.
- Injections that are too superficial (into the dermis) may result in skin ulceration and necrosis. Do not withdraw the needle from the subcutaneous fat during injection as this could increase the risk of intradermal exposure and potential skin ulceration and necrosis.
- Avoid injecting into the post-platysmal fat by injecting KYBELLA into fat tissue at the depth of approximately mid-way into the subcutaneous fat layer (Figure 2).
- If at any time resistance is met as the needle is inserted, indicating the possibility of contact with fascial or nonfat tissue, the needle must be withdrawn to an appropriate depth before the injection is administered.
- Avoid injecting into other tissues such as the muscle, salivary glands, lymph nodes; and artery or vein.
- Upon needle withdrawal, pressure may be applied to each injection site as necessary to minimize bleeding; an adhesive dressing may be applied.
- A single treatment consists of up to a maximum of 50 injections, 0.2 mL each (up to a total of 10 mL), spaced 1 cm apart.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5
WARNINGS AND PRECAUTIONS
5.1 Marginal mandibular nerve injury
Cases of marginal mandibular nerve injury, manifested as an asymmetric smile or facial muscle weakness (paresis), were reported during clinical trials. To avoid the potential for nerve injury, KYBELLA injection should not be injected into or in close proximity to the marginal mandibular branch of the facial nerve. All marginal mandibular nerve injuries reported from the trials resolved spontaneously (range 1-298 days, median 44 days).
5.2 Dysphagia
Difficulty swallowing (dysphagia) occurred in clinical trials in the setting of administration site reactions, e.g., pain, swelling, and induration of the submental area. Cases of dysphagia spontaneously resolved (range 1-81 days, median 3 days).
Subjects with current or prior history of dysphagia were excluded from clinical trials. Avoid use of KYBELLA in these patients as current or prior history of dysphagia may exacerbate the condition.
5.3 Injection site hematoma/bruising
In clinical trials, 72% of subjects treated with KYBELLA experienced injection site hematoma/bruising [see Adverse Reactions (6.1)].
KYBELLA should be used with caution in patients with bleeding abnormalities or who are currently being treated with antiplatelet or anticoagulant therapy as excessive bleeding or bruising in the treatment area may occur.
5.4 Risk of injecting in proximity to vulnerable anatomic structures
To avoid potential tissue damage, KYBELLA should not be injected into or in close proximity (1-1.5 cm) to salivary glands, lymph nodes and muscles.
Care should be taken to avoid inadvertent injection directly into an artery or a vein as it can result in vascular injury.
5.5 Injection site alopecia
Cases of injection site alopecia have been reported with the administration of KYBELLA. The onset and duration of this adverse reaction may vary among individuals and may persist. Consider withholding subsequent treatments until resolution of the adverse reaction.
5.6 Injection site ulceration, necrosis, and infection
Injections that are too superficial (into the dermis) may result in skin ulceration and necrosis [see Injection Technique (2.3)]. Cases of injection site ulceration, necrosis, and infection have been reported with the administration of KYBELLA. Some cases of injection site infection have included cellulitis and abscess requiring intravenous antibiotic treatment and incision and drainage. Do not administer KYBELLA into the affected area until complete resolution of the adverse reaction.
-
6
ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In two double-blind, placebo-controlled clinical trials 513 subjects were treated with KYBELLA injection and 506 subjects were treated with placebo. The population was 19-65 years old, 85% were women, 87% Caucasian, 8% African American. At baseline the population had a mean BMI of 29 kg/m2, moderate to severe submental convexity (graded as 2 or 3 on a 0 to 4 scale) and without excessive skin laxity. Subjects received up to 6 treatments at least 1 month apart and were followed for up to 6 months after the last received treatment.
The most commonly reported adverse reactions are listed below (Table 1).
Table 1. Adverse Reactions in the Pooled Trials 1 and 2a
Adverse reactionsKYBELLA
(N=513)
n (%)Placebo
(N=506)
n (%)Injection site reactions 492 (96%) 411 (81%) edema/swelling 448 (87%) 218 (43%) hematoma/bruising 368 (72%) 353 (70%) pain 356 (70%) 160 (32%) numbness 341 (66%) 29 (6%) erythema 136 (27%) 91 (18%) induration 120 (23%) 13 (3%) paresthesia 70 (14%) 20 (4%) nodule 68 (13%) 14 (3%) pruritus 64 (12%) 30 (6%) skin tightness 24 (5%) 6 (1%) site warmth 22 (4%) 8 (2%) nerve injury b 20 (4 %) 1 (<1%) Headache 41 (8%) 20 (4%) Oropharyngeal pain 15 (3%) 7 (1%) Hypertension 13 (3%) 7 (1%) Nausea 12 (2%) 3 (1%) Dysphagia 10 (2%) 1 (<1%) a Adverse reactions that occurred in ≥ 2% KYBELLA treated subjects and at greater incidence than placebo
b Marginal mandibular nerve paresisOther adverse reactions associated with the use of KYBELLA include: injection site hemorrhage, injection site discoloration, pre-syncope/syncope, lymphadenopathy, injection site urticaria, and neck pain.
Adverse reactions that lasted more than 30 days and occurred in more than 10% of subjects were injection site numbness (42%), injection site edema/swelling (20%), injection site pain (16%), and injection site induration (13%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of KYBELLA.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to KYBELLA exposure.
Administration site conditions: injection site ulceration, necrosis, infection, alopecia, and scarring.
Immune System Disorders: Hypersensitivity reactions including rash, urticaria, and itching.
Nervous System Disorders: Oral hypoaesthesia and oral paraesthesia.
Procedural Complications: Vascular injury due to inadvertent intravascular injection.
-
8
USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of KYBELLA injection in pregnant women to inform the drug-associated risk. In animal reproduction studies, no fetal harm was observed with the subcutaneous administration of deoxycholic acid to rats during organogenesis at doses up to 5 times the maximum recommended human dose (MRHD) of 100 mg [see Data].
The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk of major birth defects in the U.S. general population is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies.
Data
Animal Data
Embryofetal development studies have been performed in rats and rabbits using subcutaneous doses of deoxycholic acid administered during the period of organogenesis. For the basis of comparing animal to human doses, the MRHD is 1.7 mg/kg (100 mg/60 kg). No evidence of fetal harm was observed in rats at up to the highest dose tested (50 mg/kg) which is 5-fold higher than the MRHD of KYBELLA based on a mg/m2 comparison. However, missing intermediate lung lobe was noted in rabbits at all dose levels tested including the lowest dose (10 mg/kg) which is 2-fold higher than the MRHD of KYBELLA based on a mg/m2 comparison. These effects may be related to maternal toxicity, which was also seen at all dose levels tested.
8.2 Lactation
Risk Summary
There is no information available on the presence of synthetic deoxycholic acid in human milk, the effects of the drug on the breastfed infant or the effects of the drug on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for KYBELLA and any potential adverse effects on the breastfed child from KYBELLA or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness in patients below the age of 18 years have not been established and KYBELLA is not intended for use in children or adolescents.
8.5 Geriatric Use
The clinical trials of KYBELLA did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
11
DESCRIPTION
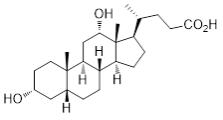
KYBELLA (deoxycholic acid) injection, 10 mg/mL is a clear colorless, sterile solution for subcutaneous use. It contains a cytolytic agent, deoxycholic acid, as the active ingredient. The chemical name of deoxycholic acid is 3α,12α-dihydroxy-5β-cholan-24-oic acid, and its molecular formula is C24H40O4, and its molecular weight is 392.57 g/mol. The chemical structure of deoxycholic acid is:
Each 2 mL vial of KYBELLA injection contains 20 mg synthetic deoxycholic acid as the active ingredient and the following inactive ingredients: benzyl alcohol (18 mg), dibasic sodium phosphate (2.84 mg), sodium chloride (8.76 mg), sodium hydroxide (2.86 mg) in water for injection, USP. Hydrochloric acid and additional sodium hydroxide are added as necessary to adjust the formulation to pH 8.3. Each vial is for single patient use.
-
12
CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
KYBELLA injection is a cytolytic drug, which when injected into tissue physically destroys the cell membrane causing lysis.
12.2 Pharmacodynamics
Cardiac Electrophysiology
At therapeutic doses, KYBELLA does not prolong the QTc interval.
12.3 Pharmacokinetics
Endogenous deoxycholic acid plasma levels are highly variable within and between individuals; most of this natural bile component is sequestered in the enterohepatic circulation loop.
Absorption and Distribution
Deoxycholic acid from KYBELLA is rapidly absorbed following subcutaneous injection. After dosing with the maximum recommended single treatment dose with KYBELLA (100 mg), maximum plasma concentrations (mean Cmax) were observed with a median Tmax of 18 minutes after injection. The mean (±SD) Cmax value was 1024 ± 304 ng/mL and was 3.2-fold higher than average Cmax values observed during a 24-hour baseline endogenous period in the absence of KYBELLA. After maximum recommended single treatment dose (100 mg), mean (±SD) deoxycholic acid exposure (AUC0-24) was 7896 ± 2269 ng.hr/mL and was 1.6-fold higher over endogenous exposure. Post-treatment deoxycholic acid plasma levels returned to the endogenous range within 24 hours. No accumulation is expected with the proposed treatment frequency.
Deoxycholic acid is extensively bound to proteins in plasma (98%).
Metabolism and Excretion
Endogenous deoxycholic acid is a product of cholesterol metabolism and is excreted intact in feces. Deoxycholic acid is not metabolized to any significant extent under normal conditions. Deoxycholic acid from KYBELLA joins the endogenous bile acid pool in the enterohepatic circulation and is excreted along with the endogenous deoxycholic acid.
In Vitro Assessment of Drug Interactions
Results from in vitro studies indicate that deoxycholic acid does not inhibit or induce human cytochrome P450 (CYP) enzymes at clinically relevant concentrations. Deoxycholic acid does not inhibit the following transporters: P-gp, BCRP, MRP4, MRP2, OATP1B1, OATP2B1, OATP1B3, OCT1, OCT2, OAT1, OAT3, NTCP, and ASBT.
Specific Populations
Hepatic Impairment
KYBELLA has not been studied in subjects with hepatic impairment. Considering the intermittent dose frequency, the small dose administered that represents approximately 3% of the total bile acid pool, and the highly variable endogenous deoxycholic acid levels, the pharmacokinetics of deoxycholic acid following KYBELLA injection is unlikely to be influenced by hepatic impairment.
Pharmacokinetic Effects of Gender
Deoxycholic acid pharmacokinetics were not influenced by gender.
-
13
NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of KYBELLA injection.
KYBELLA was negative in a battery of in vitro (Ames test and chromosomal aberration assay in human lymphocytes) and in vivo (rat erythrocyte micronucleus assay) genetic toxicology assays.
No effects on fertility were observed in male and female rats administered deoxycholic acid at subcutaneous doses up to 50 mg/kg (5 times the MRHD based on a mg/m2 comparison) once weekly prior to and during the mating period and through gestation day 7 in female rats.
-
14
CLINICAL STUDIES
Two randomized, multi-center, double-blind, placebo-controlled trials of identical design were conducted to evaluate KYBELLA injection for use in improvement in the appearance of convexity or fullness associated with submental fat. The trials enrolled healthy adults (ages 19 to 65, BMI ≤ 40 kg/m2) with moderate or severe convexity or fullness associated with submental fat (i.e., grade 2 or 3 on 5-point grading scales, where 0 = none and 4 = extreme), as judged by both clinician and subject ratings. Subjects received up to 6 treatments with KYBELLA (N=514, combined trials) or placebo (N=508, combined trials) at no less than 1 month intervals. Use of ice/cold packs, topical and/or injectable local anesthesia was allowed during the clinical trials. Injection volume was 0.2 mL per injection site, spaced 1 cm apart into the submental fat tissue, which is also expressed in dose per area as 2 mg/cm2. For each treatment session a maximum of 100 mg (10 mL) was permitted over the entire treatment area. Subjects were administered an average of 6.4 mL at the first treatment session, and subjects who received all six treatments were administered an average of 4.4 mL at the sixth treatment session. Fifty-nine percent of subjects received all six treatments.
In these trials, the mean age was 49 years and the mean BMI was 29 kg/m2. Most of the subjects were women (85%) and Caucasian (87%). At baseline, 51% of the subjects had a clinician-rated submental fat severity rating of moderate and 49% had a severe submental fat rating.
The co-primary efficacy assessments were based on at least 2-grade and at least 1-grade improvements in submental convexity or fullness on the composite of clinician-reported and patient-reported ratings of submental fat 12 weeks after final treatment. Additionally, changes in submental fat volume were evaluated in a subset of subjects (N=449, combined trials) using magnetic resonance imaging (MRI). Visual and emotional impacts of submental fat (happy, bothered, self-conscious, embarrassed, looking older or overweight) were also evaluated using a 6-question survey, with each question rated from 0 (not at all) to 10 (extremely/very much).
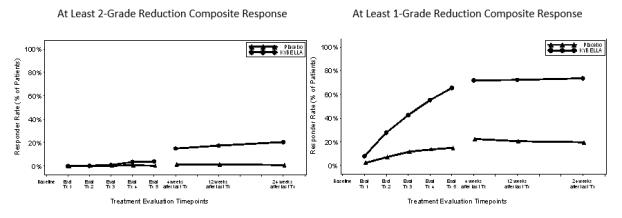
Reductions in submental fat volume were observed more frequently in the KYBELLA group compared to the placebo group as measured by the composite clinician and patient ratings (Table 2). The composite response rates by visit are presented in Figure 4.
Table 2. ≥ 2-Grade and ≥ 1-Grade Composite Clinician and Patient Response 12 Weeks After Final Treatment Trial 1 Trial 2 Endpoint KYBELLA
(N=256)Placebo
(N=250)KYBELLA
(N=258)Placebo
(N=258)2-Grade Composite Response a 13.4% <0.1% 18.6% 3.0% 1-Grade Composite Response b 70.0% 18.6% 66.5% 22.2% a At least 2 grade reduction on both the clinician-reported and patient-reported ratings of submental fat
b At least 1 grade reduction on both the clinician-reported and patient-reported ratings of submental fatFigure 4. ≥ 2-Grade and ≥ 1-Grade Composite Clinician and Patient Response
Note: Subjects were followed up 4, 12 and 24 weeks after the last treatment. Forty-one percent of subjects received fewer than 6 treatments and entered the post-treatment period earlier than Week 24.
A greater proportion of KYBELLA-treated subjects had at least a 10% reduction in submental fat volume as compared to placebo-treated subjects when evaluated by MRI (43% vs 5%, respectively).
The overall patient-reported satisfaction and self-perceived visual attributes showed greater improvement in the KYBELLA group than in the placebo group.
-
16
HOW SUPPLIED/STORAGE AND HANDLING
KYBELLA (deoxycholic acid) injection, 10 mg/mL is a clear, colorless, sterile solution supplied in 2 mL, single patient use vials in the following dispensing pack:
4 vials, NDC 61168-101-04
Store at 20°C to 25°C (68°F to 77°F); excursions are permitted between 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature].
KYBELLA has a unique hologram on the vial label. If you do not see a hologram, do not use the product and call 1-800-678-1605.
Each vial is for a single patient use. Do not dilute or mix KYBELLA with other compounds. Discard unused portion.
-
17
PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Advise patients to contact their healthcare providers if patients begin to develop signs of marginal mandibular nerve paresis (e.g., asymmetric smile, facial muscle weakness), difficulty swallowing, or worsening of existing symptoms.
Advise patients to contact their healthcare providers for development of erythema, pain, open sore(s) or drainage from the treatment area.
Distributed by: AbbVie Inc.
Manufactured for:
AbbVie Inc.
North Chicago, IL 60064
© 2023 AbbVie. All rights reserved.
KYBELLA® and its design are registered trademarks of Allergan Sales, LLC, an AbbVie company.
Patented. See www.abbvie.com/patents
v3.0USPI0101
-
PATIENT PACKAGE INSERT
Patient Information
KYBELLA® (kye be lah)
(deoxycholic acid)
injectionWhat is KYBELLA?
KYBELLA is a prescription medicine used in adults to improve the appearance and profile of moderate to severe fat below the chin (submental fat), also called “double chin.”
It is not known if KYBELLA is safe and effective for the treatment of fat outside of the submental area.
It is not known if KYBELLA is safe and effective in children under 18 years of age.Do not receive KYBELLA if you have an infection in the treatment area. Before receiving KYBELLA, tell your healthcare provider about all of your medical conditions, including if you:
- have had or plan to have surgery on your face, neck, or chin
- have had cosmetic treatments on your face, neck, or chin
- have had or have medical conditions in or near the neck area
- have had or have trouble swallowing
- have bleeding problems
- are pregnant or plan to become pregnant. It is not known if KYBELLA will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if KYBELLA passes into your breast milk.
How will I receive KYBELLA? - KYBELLA is injected into the fat under your chin (up to 50 injections under your skin) by your healthcare provider.
- KYBELLA injections may be given at least 1 month apart. You and your healthcare provider will decide how many treatments you need.
What are the possible side effects of KYBELLA?
KYBELLA can cause serious side effects, including:
-
Nerve injury in the jaw that can cause an uneven smile or facial muscle weakness.
-
Trouble swallowing.
-
Injection site problems including:
- a collection of blood under the skin (hematoma) or bruising
- damage to an artery or vein if KYBELLA is inadvertently injected into it
- hair loss
- open sores (ulcers)
- damage and tissue cell-death (necrosis) around the injection site
- infection
- a collection of blood under the skin (hematoma) or bruising
- begin to develop weakness in the muscles of your face, or your smile becomes uneven
- you have difficulty swallowing, or if any of the symptoms that you already have get worse
- develop redness, pain, open sores, or drainage at or from the treatment area
● swelling ● redness ● pain ● areas of hardness in the treatment area ● numbness These are not all of the possible side effects of KYBELLA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of KYBELLA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for more information about KYBELLA that is written for health professionals.What are the ingredients in KYBELLA?
Active ingredient: deoxycholic acid
Inactive ingredients: benzyl alcohol, dibasic sodium phosphate, hydrochloric acid, sodium chloride, sodium hydroxide and water for injection, USP.Distributed by: AbbVie Inc.
Manufactured for:
AbbVie Inc.
North Chicago, IL 60064
© 2023 AbbVie. All rights reserved.
KYBELLA® and its design are registered trademarks of Allergan Sales, LLC, an AbbVie
company.
Patented. See www.abbvie.com/patents
For more information about KYBELLA go to www.mykybella.com.

This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 3/2023
v3.0PPI0101
- have had or plan to have surgery on your face, neck, or chin
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel
NDC: 61168-101-04
kybella®
(deoxycholic acid) injection 10 mg/mL
20 mg/2 mL
(10 mg/mL)
for subcutaneous use only
Store at 20ºC to 25ºC (68ºF to 77ºF); excursions are permitted between
15ºC to 30ºC (59ºF to 86ºF) [See USP Controlled Room Temperature]
Distributed by: AbbVie, Inc.
Manufactured for: AbbVie, Inc. North Chicago, IL 60064
© 2023 AbbVie. All rights reserved. Kybella® and its design are registered
trademarks of Allergan Sales, LLC, an AbbVie Company.
Patented. See www.abbvie.com/patents.
Product of USA![NDC: 61168-101-04
kybella®
(deoxycholic acid) injection 10 mg/mL
20 mg/2 mL
(10 mg/mL)
for subcutaneous use only
Store at 20C to 25C (68F to 77F); excursions are permitted between
15C to 30C (59F to 86F) [See USP Controlled Room Temperature]
Distributed by: AbbVie, Inc.
Manufactured for: AbbVie, Inc. North Chicago, IL 60064
2023 AbbVie. All rights reserved. Kybella® and its design are registered
trademarks of Allergan Sales, LLC, an AbbVie Company.
Patented. See www.abbvie.com/patents.
Product of USA](/dailymed/image.cfm?name=kybella-07.jpg&setid=fe431ed4-ea6f-4e99-b4bc-ec25ae7b8553)
-
INGREDIENTS AND APPEARANCE
KYBELLA
deoxycholic acid injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:61168-101 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEOXYCHOLIC ACID (UNII: 005990WHZZ) (DEOXYCHOLIC ACID - UNII:005990WHZZ) DEOXYCHOLIC ACID 20 mg in 2 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) 18 mg in 2 mL SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) 2.84 mg in 2 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.76 mg in 2 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) 2.86 mg in 2 mL WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61168-101-04 4 in 1 CARTON 06/08/2015 1 NDC:61168-101-01 2 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 2 NDC:61168-101-94 4 in 1 CARTON 06/08/2015 01/30/2022 2 NDC:61168-101-91 2 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 3 NDC:61168-101-03 2 in 1 CARTON 08/01/2018 3 2 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206333 06/08/2015 Labeler - Kythera Biopharmaceuticals Inc. (800791118)