Label: GARDASIL (human papillomavirus quadrivalent- types 6, 11, 16, and 18 vaccine, recombinant injection, suspension
-
NDC Code(s):
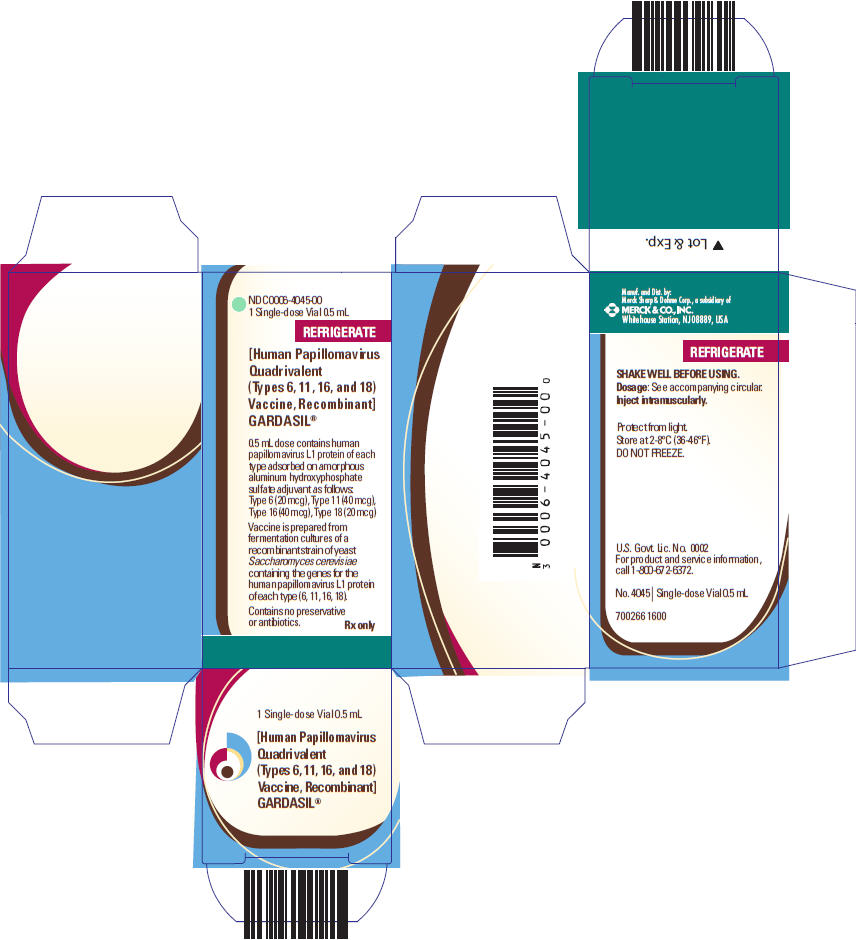
0006-4045-00,
0006-4045-01,
0006-4045-41,
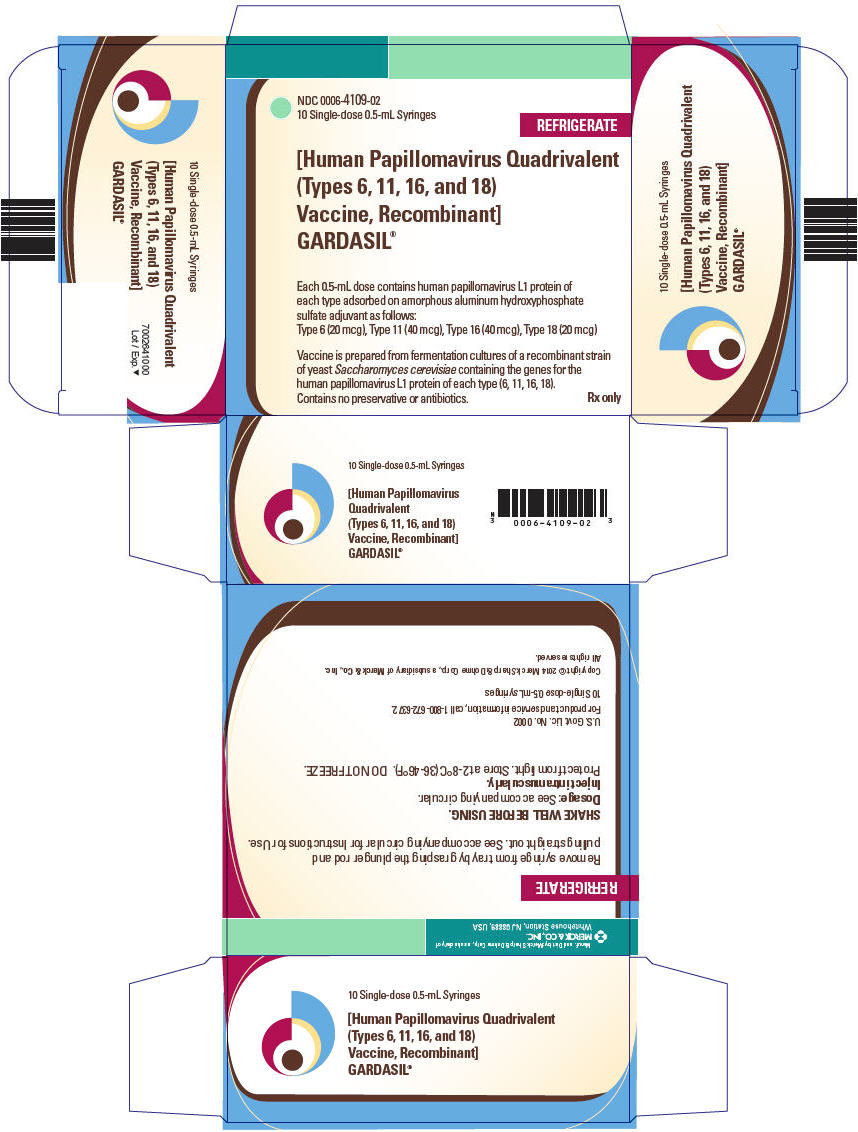
0006-4109-01, view more0006-4109-02, 0006-4109-09
- Packager: Merck Sharp & Dohme LLC
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated October 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GARDASIL safely and effectively. See full prescribing information for GARDASIL.
GARDASIL®
[Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant]
Suspension for intramuscular injection
Initial U.S. Approval: 2006INDICATIONS AND USAGE
GARDASIL is a vaccine indicated in girls and women 9 through 26 years of age for the prevention of the following diseases caused by Human Papillomavirus (HPV) types included in the vaccine:
- Cervical, vulvar, vaginal, and anal cancer caused by HPV types 16 and 18. (1.1)
- Genital warts (condyloma acuminata) caused by HPV types 6 and 11. (1.1)
And the following precancerous or dysplastic lesions caused by HPV types 6, 11, 16, and 18:
- Cervical intraepithelial neoplasia (CIN) grade 2/3 and Cervical adenocarcinoma in situ (AIS). (1.1)
- Cervical intraepithelial neoplasia (CIN) grade 1. (1.1)
- Vulvar intraepithelial neoplasia (VIN) grade 2 and grade 3. (1.1)
- Vaginal intraepithelial neoplasia (VaIN) grade 2 and grade 3. (1.1)
- Anal intraepithelial neoplasia (AIN) grades 1, 2, and 3. (1.1)
GARDASIL is indicated in boys and men 9 through 26 years of age for the prevention of the following diseases caused by HPV types included in the vaccine:
- Anal cancer caused by HPV types 16 and 18. (1.2)
- Genital warts (condyloma acuminata) caused by HPV types 6 and 11. (1.2)
And the following precancerous or dysplastic lesions caused by HPV types 6, 11, 16, and 18:
- Anal intraepithelial neoplasia (AIN) grades 1, 2, and 3. (1.2)
Limitations of GARDASIL Use and Effectiveness:
- GARDASIL does not eliminate the necessity for women to continue to undergo recommended cervical cancer screening. (1.3, 17)
- Recipients of GARDASIL should not discontinue anal cancer screening if it has been recommended by a health care provider. (1.3, 17)
- GARDASIL has not been demonstrated to provide protection against disease from vaccine and non-vaccine HPV types to which a person has previously been exposed through sexual activity. (1.3, 14.4, 14.5)
- GARDASIL is not intended to be used for treatment of active external genital lesions; cervical, vulvar, vaginal, and anal cancers; CIN; VIN; VaIN, or AIN. (1.3)
- GARDASIL has not been demonstrated to protect against diseases due to HPV types not contained in the vaccine. (1.3, 14.4, 14.5)
- Not all vulvar, vaginal, and anal cancers are caused by HPV, and GARDASIL protects only against those vulvar, vaginal, and anal cancers caused by HPV 16 and 18. (1.3)
- GARDASIL does not protect against genital diseases not caused by HPV. (1.3)
- Vaccination with GARDASIL may not result in protection in all vaccine recipients. (1.3)
- GARDASIL has not been demonstrated to prevent HPV-related CIN 2/3 or worse in women older than 26 years of age. (14.7)
DOSAGE AND ADMINISTRATION
0.5-mL suspension for intramuscular injection at the following schedule: 0, 2 months, 6 months. (2.1)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Because vaccinees may develop syncope, sometimes resulting in falling with injury, observation for 15 minutes after administration is recommended. Syncope, sometimes associated with tonic-clonic movements and other seizure-like activity, has been reported following vaccination with GARDASIL. When syncope is associated with tonic-clonic movements, the activity is usually transient and typically responds to restoring cerebral perfusion by maintaining a supine or Trendelenburg position. (5.1)
ADVERSE REACTIONS
The most common adverse reaction was headache. Common adverse reactions (frequency of at least 1.0% and greater than AAHS control or saline placebo) are fever, nausea, dizziness; and injection-site pain, swelling, erythema, pruritus, and bruising. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov .DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Safety and effectiveness of GARDASIL have not been established in the following populations:
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Girls and Women
1.2 Boys and Men
1.3 Limitations of GARDASIL Use and Effectiveness
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Method of Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Syncope
5.2 Managing Allergic Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Use with RECOMBIVAX HB
7.2 Use with Menactra and Adacel
7.3 Use with Hormonal Contraceptives
7.4 Use with Systemic Immunosuppressive Medications
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Immunocompromised Individuals
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Prophylactic Efficacy – HPV Types 6, 11, 16, and 18 in Girls and Women 16 through 26 Years of Age
14.2 Prophylactic Efficacy – HPV Types 6, 11, 16, and 18 in Boys and Men 16 through 26 Years of Age
14.3 Prophylactic Efficacy – Anal Disease Caused by HPV Types 6, 11, 16, and 18 in Boys and Men 16 through 26 Years of Age in the MSM Sub-study
14.4 Population Impact in Girls and Women 16 through 26 Years of Age
14.5 Population Impact in Boys and Men 16 through 26 Years of Age
14.6 Overall Population Impact
14.7 Studies in Women 27 through 45 Years of Age
14.8 Immunogenicity
14.9 Long-Term Follow-Up Studies
14.10 Studies with RECOMBIVAX HB [hepatitis B vaccine (recombinant)]
14.11 Studies with Menactra [Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine] and Adacel [Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap)]
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Girls and Women
GARDASIL® is a vaccine indicated in girls and women 9 through 26 years of age for the prevention of the following diseases caused by Human Papillomavirus (HPV) types included in the vaccine:
- Cervical, vulvar, vaginal, and anal cancer caused by HPV types 16 and 18
- Genital warts (condyloma acuminata) caused by HPV types 6 and 11
And the following precancerous or dysplastic lesions caused by HPV types 6, 11, 16, and 18:
- Cervical intraepithelial neoplasia (CIN) grade 2/3 and Cervical adenocarcinoma in situ (AIS)
- Cervical intraepithelial neoplasia (CIN) grade 1
- Vulvar intraepithelial neoplasia (VIN) grade 2 and grade 3
- Vaginal intraepithelial neoplasia (VaIN) grade 2 and grade 3
- Anal intraepithelial neoplasia (AIN) grades 1, 2, and 3
1.2 Boys and Men
GARDASIL is indicated in boys and men 9 through 26 years of age for the prevention of the following diseases caused by HPV types included in the vaccine:
- Anal cancer caused by HPV types 16 and 18
- Genital warts (condyloma acuminata) caused by HPV types 6 and 11
And the following precancerous or dysplastic lesions caused by HPV types 6, 11, 16, and 18:
- Anal intraepithelial neoplasia (AIN) grades 1, 2, and 3
1.3 Limitations of GARDASIL Use and Effectiveness
The health care provider should inform the patient, parent, or guardian that vaccination does not eliminate the necessity for women to continue to undergo recommended cervical cancer screening. Women who receive GARDASIL should continue to undergo cervical cancer screening per standard of care. [See Patient Counseling Information (17).]
Recipients of GARDASIL should not discontinue anal cancer screening if it has been recommended by a health care provider. [See Patient Counseling Information (17).]
GARDASIL has not been demonstrated to provide protection against disease from vaccine and non-vaccine HPV types to which a person has previously been exposed through sexual activity. [See Clinical Studies (14.4, 14.5).]
GARDASIL is not intended to be used for treatment of active external genital lesions; cervical, vulvar, vaginal, and anal cancers; CIN; VIN; VaIN; or AIN.
GARDASIL has not been demonstrated to protect against diseases due to HPV types not contained in the vaccine. [See Clinical Studies (14.4, 14.5).]
Not all vulvar, vaginal, and anal cancers are caused by HPV, and GARDASIL protects only against those vulvar, vaginal, and anal cancers caused by HPV 16 and 18.
GARDASIL does not protect against genital diseases not caused by HPV.
Vaccination with GARDASIL may not result in protection in all vaccine recipients.
GARDASIL has not been demonstrated to prevent HPV-related CIN 2/3 or worse in women older than 26 years of age. [See Clinical Studies (14.7).]
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
GARDASIL should be administered intramuscularly as a 0.5-mL dose at the following schedule: 0, 2 months, 6 months. [See Clinical Studies (14.8).]
2.2 Method of Administration
For intramuscular use only.
Shake well before use. Thorough agitation immediately before administration is necessary to maintain suspension of the vaccine. GARDASIL should not be diluted or mixed with other vaccines. After thorough agitation, GARDASIL is a white, cloudy liquid. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Do not use the product if particulates are present or if it appears discolored.
GARDASIL should be administered intramuscularly in the deltoid region of the upper arm or in the higher anterolateral area of the thigh.
Syncope has been reported following vaccination with GARDASIL and may result in falling with injury; observation for 15 minutes after administration is recommended. [See Warnings and Precautions (5.1).]
-
3 DOSAGE FORMS AND STRENGTHS
GARDASIL is a suspension for intramuscular administration available in 0.5-mL single dose vials and prefilled syringes. See Description (11) for the complete listing of ingredients.
-
4 CONTRAINDICATIONS
Hypersensitivity, including severe allergic reactions to yeast (a vaccine component), or after a previous dose of GARDASIL. [See Description (11).]
-
5 WARNINGS AND PRECAUTIONS
5.1 Syncope
Because vaccinees may develop syncope, sometimes resulting in falling with injury, observation for 15 minutes after administration is recommended. Syncope, sometimes associated with tonic-clonic movements and other seizure-like activity, has been reported following vaccination with GARDASIL. When syncope is associated with tonic-clonic movements, the activity is usually transient and typically responds to restoring cerebral perfusion by maintaining a supine or Trendelenburg position.
-
6 ADVERSE REACTIONS
Overall Summary of Adverse Reactions
Headache, fever, nausea, and dizziness; and local injection site reactions (pain, swelling, erythema, pruritus, and bruising) occurred after administration with GARDASIL.
Syncope, sometimes associated with tonic-clonic movements and other seizure-like activity, has been reported following vaccination with GARDASIL and may result in falling with injury; observation for 15 minutes after administration is recommended. [See Warnings and Precautions (5.1).]
Anaphylaxis has been reported following vaccination with GARDASIL.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
Studies in Girls and Women (9 Through 45 Years of Age) and Boys and Men (9 Through 26 Years of Age)
In 7 clinical trials (5 Amorphous Aluminum Hydroxyphosphate Sulfate [AAHS]-controlled, 1 saline placebo-controlled, and 1 uncontrolled), 18,083 individuals were administered GARDASIL or AAHS control or saline placebo on the day of enrollment, and approximately 2 and 6 months thereafter, and safety was evaluated using vaccination report cards (VRC)-aided surveillance for 14 days after each injection of GARDASIL or AAHS control or saline placebo in these individuals. The individuals who were monitored using VRC-aided surveillance included 10,088 individuals 9 through 45 years of age at enrollment who received GARDASIL and 7,995 individuals who received AAHS control or saline placebo. Few individuals (0.2%) discontinued due to adverse reactions. The race distribution of the 9- through 26-year-old girls and women in the safety population was as follows: 62.3% White; 17.6% Hispanic (Black and White); 6.8% Asian; 6.7% Other; 6.4% Black; and 0.3% American Indian. The race distribution of the 24- through 45-year-old women in the safety population of Study 6 was as follows: 20.6% White; 43.2% Hispanic (Black and White); 0.2% Other; 4.8% Black; 31.2% Asian; and 0.1% American Indian. The race distribution of the 9- through 26-year-old boys and men in the safety population was as follows: 42.0% White; 19.7% Hispanic (Black and White); 11.0% Asian; 11.2% Other; 15.9% Black; and 0.1% American Indian.
Common Injection-Site Adverse Reactions in Girls and Women 9 Through 26 Years of Age
The injection site adverse reactions that were observed among recipients of GARDASIL at a frequency of at least 1.0% and also at a greater frequency than that observed among AAHS control or saline placebo recipients are shown in Table 1.
Table 1: Injection-Site Adverse Reactions in Girls and Women 9 Through 26 Years of Age* Adverse Reaction
(1 to 5 Days Postvaccination)GARDASIL
(N = 5088)
%AAHS Control†
(N = 3470)
%Saline Placebo
(N = 320)
%Injection Site Pain 83.9 75.4 48.6 Swelling 25.4 15.8 7.3 Erythema 24.7 18.4 12.1 Pruritus 3.2 2.8 0.6 Bruising 2.8 3.2 1.6 Common Injection-Site Adverse Reactions in Boys and Men 9 Through 26 Years of Age
The injection site adverse reactions that were observed among recipients of GARDASIL at a frequency of at least 1.0% and also at a greater frequency than that observed among AAHS control or saline placebo recipients are shown in Table 2.
Evaluation of Injection-Site Adverse Reactions by Dose in Girls and Women 9 Through 26 Years of Age
An analysis of injection-site adverse reactions in girls and women by dose is shown in Table 3. Of those girls and women who reported an injection-site reaction, 94.3% judged their injection-site adverse reaction to be mild or moderate in intensity.
Table 3: Postdose Evaluation of Injection-Site Adverse Reactions in Girls and Women 9 Through 26 Years of Age (1 to 5 Days Postvaccination) GARDASIL
(% occurrence)AAHS Control*
(% occurrence)Saline Placebo
(% occurrence)Adverse Reaction Post-dose 1
N† = 5011Post-dose 2
N = 4924Post-dose 3
N = 4818Post-dose 1
N = 3410Post-dose 2
N = 3351Post-dose 3
N = 3295Post-dose 1
N = 315Post-dose 2
N = 301Post-dose 3
N = 300Pain 63.4 60.7 62.7 57.0 47.8 49.6 33.7 20.3 27.3 Mild/Moderate 62.5 59.7 61.2 56.6 47.3 48.9 33.3 20.3 27.0 Severe 0.9 1.0 1.5 0.4 0.5 0.6 0.3 0.0 0.3 Swelling‡ 10.2 12.8 15.1 8.2 7.5 7.6 4.4 3.0 3.3 Mild/Moderate 9.6 11.9 14.2 8.1 7.2 7.3 4.4 3.0 3.3 Severe 0.6 0.8 0.9 0.2 0.2 0.2 0.0 0.0 0.0 Erythema‡ 9.2 12.1 14.7 9.8 8.4 8.9 7.3 5.3 5.7 Mild/Moderate 9.0 11.7 14.3 9.5 8.4 8.8 7.3 5.3 5.7 Severe 0.2 0.3 0.4 0.3 0.1 0.1 0.0 0.0 0.0 Evaluation of Injection-Site Adverse Reactions by Dose in Boys and Men 9 Through 26 Years of Age
An analysis of injection-site adverse reactions in boys and men by dose is shown in Table 4. Of those boys and men who reported an injection-site reaction, 96.4% judged their injection-site adverse reaction to be mild or moderate in intensity.
Table 4: Postdose Evaluation of Injection-Site Adverse Reactions in Boys and Men 9 Through 26 Years of Age (1 to 5 Days Postvaccination) GARDASIL
(% occurrence)AAHS Control*
(% occurrence)Saline Placebo
(% occurrence)Adverse Reaction Post-dose 1
N† = 3003Post-dose 2
N = 2898Post-dose 3
N = 2826Post-dose 1
N = 1950Post-dose 2
N = 1854Post-dose 3
N = 1799Post-dose 1
N = 269Post-dose 2
N = 263Post-dose 3
N = 259Pain 44.7 36.9 34.4 38.4 28.2 25.8 27.5 20.5 16.2 Mild/Moderate 44.5 36.4 34.1 37.9 28.2 25.5 27.5 20.2 16.2 Severe 0.2 0.5 0.3 0.4 0.1 0.3 0.0 0.4 0.0 Swelling‡ 5.6 6.6 7.7 5.6 4.5 4.1 4.8 1.5 3.5 Mild/Moderate 5.3 6.2 7.1 5.4 4.5 4.0 4.8 1.5 3.1 Severe 0.2 0.3 0.5 0.2 0.0 0.1 0.0 0.0 0.4 Erythema‡ 7.2 8.0 8.7 8.3 6.3 5.7 7.1 5.7 5.0 Mild/Moderate 6.8 7.7 8.3 8.0 6.2 5.6 7.1 5.7 5.0 Severe 0.3 0.2 0.3 0.2 0.1 0.1 0.0 0.0 0.0 Common Systemic Adverse Reactions in Girls and Women 9 Through 26 Years of Age
Headache was the most commonly reported systemic adverse reaction in both treatment groups (GARDASIL = 28.2% and AAHS control or saline placebo = 28.4%). Fever was the next most commonly reported systemic adverse reaction in both treatment groups (GARDASIL = 13.0% and AAHS control or saline placebo = 11.2%).
Adverse reactions that were observed among recipients of GARDASIL, at a frequency of greater than or equal to 1.0% where the incidence in the GARDASIL group was greater than or equal to the incidence in the AAHS control or saline placebo group, are shown in Table 5.
Table 5: Common Systemic Adverse Reactions in Girls and Women 9 Through 26 Years of Age (GARDASIL ≥Control)* Adverse Reactions
(1 to 15 Days Postvaccination)GARDASIL
(N = 5088)
%AAHS Control† or Saline Placebo
(N = 3790)
%Pyrexia 13.0 11.2 Nausea 6.7 6.5 Dizziness 4.0 3.7 Diarrhea 3.6 3.5 Vomiting 2.4 1.9 Cough 2.0 1.5 Toothache 1.5 1.4 Upper respiratory tract infection 1.5 1.5 Malaise 1.4 1.2 Arthralgia 1.2 0.9 Insomnia 1.2 0.9 Nasal congestion 1.1 0.9 Common Systemic Adverse Reactions in Boys and Men 9 Through 26 Years of Age
Headache was the most commonly reported systemic adverse reaction in both treatment groups (GARDASIL = 12.3% and AAHS control or saline placebo = 11.2%). Fever was the next most commonly reported systemic adverse reaction in both treatment groups (GARDASIL = 8.3% and AAHS control or saline placebo = 6.5%).
Adverse reactions that were observed among recipients of GARDASIL, at a frequency of greater than or equal to 1.0% where the incidence in the group that received GARDASIL was greater than or equal to the incidence in the AAHS control or saline placebo group, are shown in Table 6.
Table 6: Common Systemic Adverse Reactions in Boys and Men 9 Through 26 Years of Age (GARDASIL ≥Control)* Adverse Reactions
(1 to 15 Days Postvaccination)GARDASIL
(N = 3093)
%AAHS Control† or Saline Placebo
(N = 2303)
%Headache 12.3 11.2 Pyrexia 8.3 6.5 Oropharyngeal pain 2.8 2.1 Diarrhea 2.7 2.2 Nasopharyngitis 2.6 2.6 Nausea 2.0 1.0 Upper respiratory tract infection 1.5 1.0 Abdominal pain upper 1.4 1.4 Myalgia 1.3 0.7 Dizziness 1.2 0.9 Vomiting 1.0 0.8 Evaluation of Fever by Dose in Girls and Women 9 Through 26 Years of Age
An analysis of fever in girls and women by dose is shown in Table 7.
Table 7: Postdose Evaluation of Fever in Girls and Women 9 Through 26 Years of Age (1 to 5 Days Postvaccination) GARDASIL
(% occurrence)AAHS Control* or Saline Placebo
(% occurrence)Temperature
(°F)Postdose 1
N† = 4945Postdose 2
N = 4804Postdose 3
N = 4671Postdose 1
N = 3681Postdose 2
N = 3564Postdose 3
N = 3467≥100 to <102 3.7 4.1 4.4 3.1 3.8 3.6 ≥102 0.3 0.5 0.5 0.2 0.4 0.5 Evaluation of Fever by Dose in Boys and Men 9 Through 26 Years of Age
An analysis of fever in boys and men by dose is shown in Table 8.
Table 8: Postdose Evaluation of Fever in Boys and Men 9 Through 26 Years of Age (1 to 5 Days Postvaccination) GARDASIL
(% occurrence)AAHS Control* or Saline Placebo
(% occurrence)Temperature
(°F)Postdose 1
N† = 2972Postdose 2
N = 2849Postdose 3
N = 2792Postdose 1
N = 2194Postdose 2
N = 2079Postdose 3
N = 2046≥100 to <102 2.4 2.5 2.3 2.1 2.2 1.6 ≥102 0.6 0.5 0.5 0.5 0.3 0.3 Serious Adverse Reactions in the Entire Study Population
Across the clinical studies, 258 individuals (GARDASIL N = 128 or 0.8%; placebo N = 130 or 1.0%) out of 29,323 (GARDASIL N = 15,706; AAHS control N = 13,023; or saline placebo N = 594) individuals (9- through 45-year-old girls and women; and 9- through 26-year-old boys and men) reported a serious systemic adverse reaction.
Of the entire study population (29,323 individuals), 0.04% of the reported serious systemic adverse reactions were judged to be vaccine related by the study investigator. The most frequently (frequency of 4 cases or greater with either GARDASIL, AAHS control, saline placebo, or the total of all three) reported serious systemic adverse reactions, regardless of causality, were:
Headache [0.02% GARDASIL (3 cases) vs. 0.02% AAHS control (2 cases)],
Gastroenteritis [0.02% GARDASIL (3 cases) vs. 0.02% AAHS control (2 cases)],
Appendicitis [0.03% GARDASIL (5 cases) vs. 0.01% AAHS control (1 case)],
Pelvic inflammatory disease [0.02% GARDASIL (3 cases) vs. 0.03% AAHS control (4 cases)],
Urinary tract infection [0.01% GARDASIL (2 cases) vs. 0.02% AAHS control (2 cases)],
Pneumonia [0.01% GARDASIL (2 cases) vs. 0.02% AAHS control (2 cases)],
Pyelonephritis [0.01% GARDASIL (2 cases) vs. 0.02% AAHS control (3 cases)],
Pulmonary embolism [0.01% GARDASIL (2 cases) vs. 0.02% AAHS control (2 cases)].One case (0.006% GARDASIL; 0.0% AAHS control or saline placebo) of bronchospasm; and 2 cases (0.01% GARDASIL; 0.0% AAHS control or saline placebo) of asthma were reported as serious systemic adverse reactions that occurred following any vaccination visit.
In addition, there was 1 individual in the clinical trials, in the group that received GARDASIL, who reported two injection-site serious adverse reactions (injection-site pain and injection-site joint movement impairment).
Deaths in the Entire Study Population
Across the clinical studies, 40 deaths (GARDASIL N = 21 or 0.1%; placebo N = 19 or 0.1%) were reported in 29,323 (GARDASIL N = 15,706; AAHS control N = 13,023, saline placebo N = 594) individuals (9- through 45-year-old girls and women; and 9- through 26-year-old boys and men). The events reported were consistent with events expected in healthy adolescent and adult populations. The most common cause of death was motor vehicle accident (5 individuals who received GARDASIL and 4 individuals who received AAHS control), followed by drug overdose/suicide (2 individuals who received GARDASIL and 6 individuals who received AAHS control), gunshot wound (1 individual who received GARDASIL and 3 individuals who received AAHS control), and pulmonary embolus/deep vein thrombosis (1 individual who received GARDASIL and 1 individual who received AAHS control). In addition, there were 2 cases of sepsis, 1 case of pancreatic cancer, 1 case of arrhythmia, 1 case of pulmonary tuberculosis, 1 case of hyperthyroidism, 1 case of post-operative pulmonary embolism and acute renal failure, 1 case of traumatic brain injury/cardiac arrest, 1 case of systemic lupus erythematosus, 1 case of cerebrovascular accident, 1 case of breast cancer, and 1 case of nasopharyngeal cancer in the group that received GARDASIL; 1 case of asphyxia, 1 case of acute lymphocytic leukemia, 1 case of chemical poisoning, and 1 case of myocardial ischemia in the AAHS control group; and 1 case of medulloblastoma in the saline placebo group.
Systemic Autoimmune Disorders in Girls and Women 9 Through 26 Years of Age
In the clinical studies, 9- through 26-year-old girls and women were evaluated for new medical conditions that occurred over the course of follow-up. New medical conditions potentially indicative of a systemic autoimmune disorder seen in the group that received GARDASIL or AAHS control or saline placebo are shown in Table 9. This population includes all girls and women who received at least one dose of GARDASIL or AAHS control or saline placebo, and had safety data available.
Table 9: Summary of Girls and Women 9 Through 26 Years of Age Who Reported an Incident Condition Potentially Indicative of a Systemic Autoimmune Disorder After Enrollment in Clinical Trials of GARDASIL, Regardless of Causality Conditions GARDASIL
(N = 10,706)AAHS Control* or Saline Placebo
(N = 9412)n (%) n (%) N = Number of individuals enrolled n = Number of individuals with specific new Medical Conditions NOTE: Although an individual may have had two or more new Medical Conditions, the individual is counted only once within a category. The same individual may appear in different categories. - *
- AAHS Control = Amorphous Aluminum Hydroxyphosphate Sulfate
- †
- Arthralgia/Arthritis/Arthropathy includes the following terms: Arthralgia, Arthritis, Arthritis reactive, and Arthropathy
- ‡
- Hyperthyroidism includes the following terms: Basedow's disease, Goiter, Toxic nodular goiter, and Hyperthyroidism
- §
- Hypothyroidism includes the following terms: Hypothyroidism and thyroiditis
- ¶
- Inflammatory bowel disease includes the following terms: Colitis ulcerative, Crohn's disease, and Inflammatory bowel disease
- #
- Nephritis includes the following terms: Nephritis, Glomerulonephritis minimal lesion, Glomerulonephritis proliferative
- Þ
- Pigmentation disorder includes the following terms: Pigmentation disorder, Skin depigmentation, and Vitiligo
- ß
- Psoriasis includes the following terms: Psoriasis, Pustular psoriasis, and Psoriatic arthropathy
- à
- Rheumatoid arthritis includes juvenile rheumatoid arthritis. One woman counted in the rheumatoid arthritis group reported rheumatoid arthritis as an adverse experience at Day 130.
Arthralgia/Arthritis/Arthropathy† 120 (1.1) 98 (1.0) Autoimmune Thyroiditis 4 (0.0) 1 (0.0) Celiac Disease 10 (0.1) 6 (0.1) Diabetes Mellitus Insulin-dependent 2 (0.0) 2 (0.0) Erythema Nodosum 2 (0.0) 4 (0.0) Hyperthyroidism‡ 27 (0.3) 21 (0.2) Hypothyroidism§ 35 (0.3) 38 (0.4) Inflammatory Bowel Disease¶ 7 (0.1) 10 (0.1) Multiple Sclerosis 2 (0.0) 4 (0.0) Nephritis# 2 (0.0) 5 (0.1) Optic Neuritis 2 (0.0) 0 (0.0) Pigmentation DisorderÞ 4 (0.0) 3 (0.0) Psoriasisß 13 (0.1) 15 (0.2) Raynaud's Phenomenon 3 (0.0) 4 (0.0) Rheumatoid Arthritisà 6 (0.1) 2 (0.0) Scleroderma/Morphea 2 (0.0) 1 (0.0) Stevens-Johnson Syndrome 1 (0.0) 0 (0.0) Systemic Lupus Erythematosus 1 (0.0) 3 (0.0) Uveitis 3 (0.0) 1 (0.0) All Conditions 245 (2.3) 218 (2.3) Systemic Autoimmune Disorders in Boys and Men 9 Through 26 Years of Age
In the clinical studies, 9- through 26-year-old boys and men were evaluated for new medical conditions that occurred over the course of follow-up. New medical conditions potentially indicative of a systemic autoimmune disorder seen in the group that received GARDASIL or AAHS control or saline placebo are shown in Table 10. This population includes all boys and men who received at least one dose of GARDASIL or AAHS control or saline placebo, and had safety data available.
Table 10: Summary of Boys and Men 9 Through 26 Years of Age Who Reported an Incident Condition Potentially Indicative of a Systemic Autoimmune Disorder After Enrollment in Clinical Trials of GARDASIL, Regardless of Causality Conditions GARDASIL
(N = 3093)AAHS Control* or Saline Placebo
(N = 2303)n (%) n (%) N = Number of individuals who received at least one dose of either vaccine or placebo n = Number of individuals with specific new Medical Conditions NOTE: Although an individual may have had two or more new Medical Conditions, the individual is counted only once within a category. The same individual may appear in different categories. Alopecia Areata 2 (0.1) 0 (0.0) Ankylosing Spondylitis 1 (0.0) 2 (0.1) Arthralgia/Arthritis/Reactive Arthritis 30 (1.0) 17 (0.7) Autoimmune Thrombocytopenia 1 (0.0) 0 (0.0) Diabetes Mellitus Type 1 3 (0.1) 2 (0.1) Hyperthyroidism 0 (0.0) 1 (0.0) Hypothyroidism† 3 (0.1) 0 (0.0) Inflammatory Bowel Disease‡ 1 (0.0) 2 (0.1) Myocarditis 1 (0.0) 1 (0.0) Proteinuria 1 (0.0) 0 (0.0) Psoriasis 0 (0.0) 4 (0.2) Skin Depigmentation 1 (0.0) 0 (0.0) Vitiligo 2 (0.1) 5 (0.2) All Conditions 46 (1.5) 34 (1.5) Safety in Concomitant Use with RECOMBIVAX HB® [hepatitis B vaccine (recombinant)] in Girls and Women 16 Through 23 Years of Age
The safety of GARDASIL when administered concomitantly with RECOMBIVAX HB® [hepatitis B vaccine (recombinant)] was evaluated in an AAHS-controlled study of 1871 girls and women with a mean age of 20.4 years [see Clinical Studies (14.10)]. The race distribution of the study individuals was as follows: 61.6% White; 23.8% Other; 11.9% Black; 1.6% Hispanic (Black and White); 0.8% Asian; and 0.3% American Indian. The rates of systemic and injection-site adverse reactions were similar among girls and women who received concomitant vaccination as compared with those who received GARDASIL or RECOMBIVAX HB [hepatitis B vaccine (recombinant)].
Safety in Concomitant Use with Menactra [Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine] and Adacel [Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap)]
The safety of GARDASIL when administered concomitantly with Menactra [Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine] and Adacel [Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap)] was evaluated in a randomized study of 1040 boys and girls with a mean age of 12.6 years [see Clinical Studies (14.11)]. The race distribution of the study subjects was as follows: 77.7% White; 1.4% Multi-racial; 12.3% Black; 6.8% Hispanic (Black and White); 1.2% Asian; 0.4% American Indian, and 0.2% Indian.
There was an increase in injection-site swelling reported at the injection site for GARDASIL (concomitant = 10.9%, non-concomitant = 6.9%) when GARDASIL was administered concomitantly with Menactra and Adacel as compared to non-concomitant (separated by 1 month) vaccination. The majority of injection-site swelling adverse experiences were reported as being mild to moderate in intensity.
6.2 Postmarketing Experience
The following adverse events have been spontaneously reported during post-approval use of GARDASIL. Because these events were reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or to establish a causal relationship to vaccine exposure.
Blood and lymphatic system disorders: Autoimmune hemolytic anemia, idiopathic thrombocytopenic purpura, lymphadenopathy.
Respiratory, thoracic and mediastinal disorders: Pulmonary embolus.
Gastrointestinal disorders: Nausea, pancreatitis, vomiting.
General disorders and administration site conditions: Asthenia, chills, death, fatigue, malaise.
Immune system disorders: Autoimmune diseases, hypersensitivity reactions including anaphylactic/anaphylactoid reactions, bronchospasm, and urticaria.
Musculoskeletal and connective tissue disorders: Arthralgia, myalgia.
Nervous system disorders: Acute disseminated encephalomyelitis, dizziness, Guillain-Barré syndrome, headache, motor neuron disease, paralysis, seizures, syncope (including syncope associated with tonic-clonic movements and other seizure-like activity) sometimes resulting in falling with injury, transverse myelitis.
Infections and infestations: Cellulitis.
Vascular disorders: Deep venous thrombosis.
-
7 DRUG INTERACTIONS
7.1 Use with RECOMBIVAX HB
Results from clinical studies indicate that GARDASIL may be administered concomitantly (at a separate injection site) with RECOMBIVAX HB [hepatitis B vaccine (recombinant)] [see Clinical Studies (14.10)].
7.2 Use with Menactra and Adacel
Results from clinical studies indicate that GARDASIL may be administered concomitantly (at a separate injection site) with Menactra [Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine] and Adacel [Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap)] [see Clinical Studies (14.11)].
7.3 Use with Hormonal Contraceptives
In clinical studies of 16- through 26-year-old women, 13,912 (GARDASIL N = 6952; AAHS control or saline placebo N = 6960) who had post-Month 7 follow-up used hormonal contraceptives for a total of 33,859 person-years (65.8% of the total follow-up time in the studies).
In one clinical study of 24- through 45-year-old women, 1357 (GARDASIL N = 690; AAHS control N = 667) who had post-Month 7 follow-up used hormonal contraceptives for a total of 3400 person-years (31.5% of the total follow-up time in the study). Use of hormonal contraceptives or lack of use of hormonal contraceptives among study participants did not impair the immune response in the per protocol immunogenicity (PPI) population.
7.4 Use with Systemic Immunosuppressive Medications
Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs, and corticosteroids (used in greater than physiologic doses), may reduce the immune responses to vaccines [see Use in Specific Populations (8.6)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
Reproduction studies have been performed in female rats at doses equivalent to the recommended human dose and have revealed no evidence of impaired female fertility or harm to the fetus due to GARDASIL. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human responses, GARDASIL should be used during pregnancy only if clearly needed.
An evaluation of the effect of GARDASIL on embryo-fetal, pre- and postweaning development was conducted using rats. One group of rats was administered GARDASIL twice prior to gestation, during the period of organogenesis (gestation Day 6) and on lactation Day 7. A second group of pregnant rats was administered GARDASIL during the period of organogenesis (gestation Day 6) and on lactation Day 7 only. GARDASIL was administered at 0.5 mL/rat/occasion (120 mcg total protein which is equivalent to the recommended human dose) by intramuscular injection. No adverse effects on mating, fertility, pregnancy, parturition, lactation, embryo-fetal or pre- and postweaning development were observed. There were no vaccine-related fetal malformations or other evidence of teratogenesis noted in this study. In addition, there were no treatment-related effects on developmental signs, behavior, reproductive performance, or fertility of the offspring.
Clinical Studies in Humans
In clinical studies, women underwent urine pregnancy testing prior to administration of each dose of GARDASIL. Women who were found to be pregnant before completion of a 3-dose regimen of GARDASIL were instructed to defer completion of their vaccination regimen until resolution of the pregnancy.
GARDASIL is not indicated for women 27 years of age or older. However, safety data in women 16 through 45 years of age was collected, and 3819 women (GARDASIL N = 1894 vs. AAHS control or saline placebo N = 1925) reported at least 1 pregnancy each.
The overall proportions of pregnancies that resulted in an adverse outcome, defined as the combined numbers of spontaneous abortion, late fetal death, and congenital anomaly cases out of the total number of pregnancy outcomes for which an outcome was known (and excluding elective terminations), were 22.6% (446/1973) in women who received GARDASIL and 23.1% (460/1994) in women who received AAHS control or saline placebo.
Overall, 55 and 65 women in the group that received GARDASIL or AAHS control or saline placebo, respectively (2.9% and 3.4% of all women who reported a pregnancy in the respective vaccination groups), experienced a serious adverse reaction during pregnancy. The most common events reported were conditions that can result in Caesarean section (e.g., failure of labor, malpresentation, cephalopelvic disproportion), premature onset of labor (e.g., threatened abortions, premature rupture of membranes), and pregnancy-related medical problems (e.g., pre-eclampsia, hyperemesis). The proportions of pregnant women who experienced such events were comparable between the groups receiving GARDASIL and AAHS control or saline placebo.
There were 45 cases of congenital anomaly in pregnancies that occurred in women who received GARDASIL and 34 cases of congenital anomaly in pregnancies that occurred in women who received AAHS control or saline placebo.
Further sub-analyses were conducted to evaluate pregnancies with estimated onset within 30 days or more than 30 days from administration of a dose of GARDASIL or AAHS control or saline placebo. For pregnancies with estimated onset within 30 days of vaccination, 5 cases of congenital anomaly were observed in the group that received GARDASIL compared to 1 case of congenital anomaly in the group that received AAHS control or saline placebo. The congenital anomalies seen in pregnancies with estimated onset within 30 days of vaccination included pyloric stenosis, congenital megacolon, congenital hydronephrosis, hip dysplasia, and club foot. Conversely, in pregnancies with onset more than 30 days following vaccination, 40 cases of congenital anomaly were observed in the group that received GARDASIL compared with 33 cases of congenital anomaly in the group that received AAHS control or saline placebo.
Women who receive GARDASIL during pregnancy are encouraged to contact Merck Sharp & Dohme LLC at 1-877-888-4231 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov .
8.3 Nursing Mothers
Women 16 Through 45 Years of Age
It is not known whether GARDASIL is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when GARDASIL is administered to a nursing woman.
GARDASIL or AAHS control were given to a total of 1133 women (vaccine N = 582, AAHS control N = 551) during the relevant Phase 3 clinical studies.
Overall, 27 and 13 infants of women who received GARDASIL or AAHS control, respectively (representing 4.6% and 2.4% of the total number of women who were breast-feeding during the period in which they received GARDASIL or AAHS control, respectively), experienced a serious adverse reaction.
In a post-hoc analysis of clinical studies, a higher number of breast-feeding infants (n = 7) whose mothers received GARDASIL had acute respiratory illnesses within 30 days post vaccination of the mother as compared to infants (n = 2) whose mothers received AAHS control.
8.4 Pediatric Use
Safety and effectiveness have not been established in pediatric patients below 9 years of age.
8.5 Geriatric Use
The safety and effectiveness of GARDASIL have not been evaluated in a geriatric population, defined as individuals aged 65 years and over.
8.6 Immunocompromised Individuals
The immunologic response to GARDASIL may be diminished in immunocompromised individuals [see Drug Interactions (7.4)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
GARDASIL, Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant, is a non-infectious recombinant quadrivalent vaccine prepared from the purified virus-like particles (VLPs) of the major capsid (L1) protein of HPV Types 6, 11, 16, and 18. The L1 proteins are produced by separate fermentations in recombinant Saccharomyces cerevisiae and self-assembled into VLPs. The fermentation process involves growth of S. cerevisiae on chemically-defined fermentation media which include vitamins, amino acids, mineral salts, and carbohydrates. The VLPs are released from the yeast cells by cell disruption and purified by a series of chemical and physical methods. The purified VLPs are adsorbed on preformed aluminum-containing adjuvant (Amorphous Aluminum Hydroxyphosphate Sulfate). The quadrivalent HPV VLP vaccine is a sterile liquid suspension that is prepared by combining the adsorbed VLPs of each HPV type and additional amounts of the aluminum-containing adjuvant and the final purification buffer.
GARDASIL is a sterile suspension for intramuscular administration. Each 0.5-mL dose contains approximately 20 mcg of HPV 6 L1 protein, 40 mcg of HPV 11 L1 protein, 40 mcg of HPV 16 L1 protein, and 20 mcg of HPV 18 L1 protein.
Each 0.5-mL dose of the vaccine contains approximately 225 mcg of aluminum (as Amorphous Aluminum Hydroxyphosphate Sulfate adjuvant), 9.56 mg of sodium chloride, 0.78 mg of L-histidine, 50 mcg of polysorbate 80, 35 mcg of sodium borate, <7 mcg yeast protein/dose, and water for injection. The product does not contain a preservative or antibiotics.
After thorough agitation, GARDASIL is a white, cloudy liquid.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
HPV only infects human beings. Animal studies with analogous animal papillomaviruses suggest that the efficacy of L1 VLP vaccines may involve the development of humoral immune responses. Human beings develop a humoral immune response to the vaccine, although the exact mechanism of protection is unknown.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
GARDASIL has not been evaluated for the potential to cause carcinogenicity or genotoxicity.
GARDASIL administered to female rats at a dose of 120 mcg total protein, which is equivalent to the recommended human dose, had no effects on mating performance, fertility, or embryonic/fetal survival.
The effect of GARDASIL on male fertility has been studied in male rats at an intramuscular dose of 0.5 mL/rat/occasion (120 mcg total protein which is equivalent to the recommended human dose). One group of male rats was administered GARDASIL once, 3 days prior to cohabitation, and a second group of male rats was administered GARDASIL three times, at 6 weeks, 3 weeks, and 3 days prior to cohabitation. There were no treatment-related effects on reproductive performance including fertility, sperm count, and sperm motility. There were no treatment-related gross or histomorphologic and weight changes on the testes.
-
14 CLINICAL STUDIES
CIN 2/3 and AIS are the immediate and necessary precursors of squamous cell carcinoma and adenocarcinoma of the cervix, respectively. Their detection and removal has been shown to prevent cancer; thus, they serve as surrogate markers for prevention of cervical cancer. In the clinical studies in girls and women aged 16 through 26 years, cases of CIN 2/3 and AIS were the efficacy endpoints to assess prevention of cervical cancer. In addition, cases of VIN 2/3 and VaIN 2/3 were the efficacy endpoints to assess prevention of HPV-related vulvar and vaginal cancers, and observations of external genital lesions were the efficacy endpoints for the prevention of genital warts.
In clinical studies in boys and men aged 16 through 26 years, efficacy was evaluated using the following endpoints: external genital warts and penile/perineal/perianal intraepithelial neoplasia (PIN) grades 1/2/3 or penile/perineal/perianal cancer. In addition, cases of AIN grades 1/2/3 and anal cancer made up the composite efficacy endpoint used to assess prevention of HPV-related anal cancer.
Anal HPV infection, AIN, and anal cancer were not endpoints in the studies conducted in women. The similarity of HPV-related anal disease in men and women supports bridging the indication of prevention of AIN and anal cancer to women.
Efficacy was assessed in 6 AAHS-controlled, double-blind, randomized Phase 2 and 3 clinical studies. The first Phase 2 study evaluated the HPV 16 component of GARDASIL (Study 1, N = 2391 16- through 26-year-old girls and women) and the second evaluated all components of GARDASIL (Study 2, N = 551 16- through 26-year-old girls and women). Two Phase 3 studies evaluated GARDASIL in 5442 (Study 3) and 12,157 (Study 4) 16- through 26-year-old girls and women. A third Phase 3 study, Study 5, evaluated GARDASIL in 4055 16- through 26-year-old boys and men, including a subset of 598 (GARDASIL = 299; placebo = 299) men who self-identified as having sex with men (MSM population). A fourth Phase 3 study, Study 6, evaluated GARDASIL in 3817 24- through 45-year-old women. Together, these six studies evaluated 28,413 individuals (20,541 girls and women 16 through 26 years of age at enrollment with a mean age of 20.0 years, 4055 boys and men 16 through 26 years of age at enrollment with a mean age of 20.5 years, and 3817 women 24 through 45 years of age at enrollment with a mean age of 34.3 years). The race distribution of the 16- through 26-year-old girls and women in the clinical trials was as follows: 70.4% White; 12.2% Hispanic (Black and White); 8.8% Other; 4.6% Black; 3.8% Asian; and 0.2% American Indian. The race distribution of the 16- through 26-year-old boys and men in the clinical trials was as follows: 35.2% White; 20.5% Hispanic (Black and White); 14.4% Other; 19.8% Black; 10.0% Asian; and 0.1% American Indian. The race distribution of the 24- through 45-year-old women in the clinical trials was as follows: 20.6% White; 43.2% Hispanic (Black and White); 0.2% Other; 4.8% Black; 31.2% Asian; and 0.1% American Indian.
The median duration of follow-up was 4.0, 3.0, 3.0, 3.0, 2.3, and 4.0 years for Study 1, Study 2, Study 3, Study 4, Study 5, and Study 6, respectively. Individuals received vaccine or AAHS control on the day of enrollment and 2 and 6 months thereafter. Efficacy was analyzed for each study individually and for all studies in girls and women combined according to a prospective clinical plan.
Overall, 73% of 16- through 26-year-old girls and women, 67% of 24- through 45-year-old women, and 83% of 16- through 26-year-old boys and men were naïve (i.e., PCR [Polymerase Chain Reaction] negative and seronegative for all 4 vaccine HPV types) to all 4 vaccine HPV types at enrollment.
A total of 27% of 16- through 26-year-old girls and women, 33% of 24- through 45-year-old women, and 17% of 16- through 26-year-old boys and men had evidence of prior exposure to or ongoing infection with at least 1 of the 4 vaccine HPV types. Among these individuals, 74% of 16- through 26-year-old girls and women, 71% of 24- through 45-year-old women, and 78% of 16- through 26-year-old boys and men had evidence of prior exposure to or ongoing infection with only 1 of the 4 vaccine HPV types and were naïve (PCR negative and seronegative) to the remaining 3 types.
In 24- through 45-year-old individuals, 0.4% had been exposed to all 4 vaccine HPV types.
In individuals who were naïve (PCR negative and seronegative) to all 4 vaccine HPV types, CIN, genital warts, VIN, VaIN, PIN, and persistent infection caused by any of the 4 vaccine HPV types were counted as endpoints.
Among individuals who were positive (PCR positive and/or seropositive) for a vaccine HPV type at Day 1, endpoints related to that type were not included in the analyses of prophylactic efficacy. Endpoints related to the remaining types for which the individual was naïve (PCR negative and seronegative) were counted.
For example, in individuals who were HPV 18 positive (PCR positive and/or seropositive) at Day 1, lesions caused by HPV 18 were not counted in the prophylactic efficacy evaluations. Lesions caused by HPV 6, 11, and 16 were included in the prophylactic efficacy evaluations. The same approach was used for the other types.
14.1 Prophylactic Efficacy – HPV Types 6, 11, 16, and 18 in Girls and Women 16 through 26 Years of Age
GARDASIL was administered without prescreening for presence of HPV infection and the efficacy trials allowed enrollment of girls and women regardless of baseline HPV status (i.e., PCR status or serostatus). Girls and women with current or prior HPV infection with an HPV type contained in the vaccine were not eligible for prophylactic efficacy evaluations for that type.
The primary analyses of efficacy with respect to HPV types 6, 11, 16, and 18 were conducted in the per-protocol efficacy (PPE) population, consisting of girls and women who received all 3 vaccinations within 1 year of enrollment, did not have major deviations from the study protocol, and were naïve (PCR negative in cervicovaginal specimens and seronegative) to the relevant HPV type(s) (Types 6, 11, 16, and 18) prior to dose 1 and through 1 month Postdose 3 (Month 7). Efficacy was measured starting after the Month 7 visit.
GARDASIL was efficacious in reducing the incidence of CIN (any grade including CIN 2/3); AIS; genital warts; VIN (any grade); and VaIN (any grade) related to vaccine HPV types 6, 11, 16, or 18 in those who were PCR negative and seronegative at baseline (Table 11).
In addition, girls and women who were already infected with 1 or more vaccine-related HPV types prior to vaccination were protected from precancerous cervical lesions and external genital lesions caused by the other vaccine HPV types.
Table 11: Analysis of Efficacy of GARDASIL in the PPE* Population† of 16- Through 26-Year-Old Girls and Women for Vaccine HPV Types Population GARDASIL AAHS Control % Efficacy (95% CI) N Number of cases N Number of cases N = Number of individuals with at least 1 follow-up visit after Month 7 CI = Confidence Interval Note 1: Point estimates and confidence intervals are adjusted for person-time of follow-up. Note 2: The first analysis in the table (i.e., HPV 16- or 18-related CIN 2/3, AIS or worse) was the primary endpoint of the vaccine development plan. Note 3: Table 11 does not include cases due to non-vaccine HPV types. AAHS Control = Amorphous Aluminum Hydroxyphosphate Sulfate - *
- The PPE population consisted of individuals who received all 3 vaccinations within 1 year of enrollment, did not have major deviations from the study protocol, and were naïve (PCR negative and seronegative) to the relevant HPV type(s) (Types 6, 11, 16, and 18) prior to dose 1 and through 1 month postdose 3 (Month 7).
- †
- See Table 14 for analysis of vaccine impact in the general population.
- ‡
- Evaluated only the HPV 16 L1 VLP vaccine component of GARDASIL
- §
- Analyses of the combined trials were prospectively planned and included the use of similar study entry criteria.
HPV 16- or 18-related CIN 2/3 or AIS Study 1‡ 755 0 750 12 100.0 (65.1, 100.0) Study 2 231 0 230 1 100.0 (-3744.9, 100.0) Study 3 2201 0 2222 36 100.0 (89.2, 100.0) Study 4 5306 2 5262 63 96.9 (88.2, 99.6) Combined Protocols§ 8493 2 8464 112 98.2 (93.5, 99.8) HPV 16-related CIN 2/3 or AIS Combined Protocols§ 7402 2 7205 93 97.9 (92.3, 99.8) HPV 18-related CIN 2/3 or AIS Combined Protocols§ 7382 0 7316 29 100.0 (86.6, 100.0) HPV 16- or 18-related VIN 2/3 Study 2 231 0 230 0 Not calculated Study 3 2219 0 2239 6 100.0 (14.4, 100.0) Study 4 5322 0 5275 4 100.0 (-50.3, 100.0) Combined Protocols§ 7772 0 7744 10 100.0 (55.5, 100.0) HPV 16- or 18-related VaIN 2/3 Study 2 231 0 230 0 Not calculated Study 3 2219 0 2239 5 100.0 (-10.1, 100.0) Study 4 5322 0 5275 4 100.0 (-50.3, 100.0) Combined Protocols§ 7772 0 7744 9 100.0 (49.5, 100.0) HPV 6-, 11-, 16-, or 18-related CIN (CIN 1, CIN 2/3) or AIS Study 2 235 0 233 3 100.0 (-138.4, 100.0) Study 3 2241 0 2258 77 100.0 (95.1, 100.0) Study 4 5388 9 5374 145 93.8 (88.0, 97.2) Combined Protocols§ 7864 9 7865 225 96.0 (92.3, 98.2) HPV 6-, 11-, 16-, or 18-related Genital Warts Study 2 235 0 233 3 100.0 (-139.5, 100.0) Study 3 2261 0 2279 58 100.0 (93.5, 100.0) Study 4 5404 2 5390 132 98.5 (94.5, 99.8) Combined Protocols§ 7900 2 7902 193 99.0 (96.2, 99.9) HPV 6- and 11-related Genital Warts Combined Protocols§ 6932 2 6856 189 99.0 (96.2, 99.9) Prophylactic efficacy against overall cervical and genital disease related to HPV 6, 11, 16, and 18 in an extension phase of Study 2, that included data through Month 60, was noted to be 100% (95% CI: 12.3%, 100.0%) among girls and women in the per protocol population naïve to the relevant HPV types.
GARDASIL was efficacious against HPV disease caused by HPV types 6, 11, 16, and 18 in girls and women who were naïve for those specific HPV types at baseline.
14.2 Prophylactic Efficacy – HPV Types 6, 11, 16, and 18 in Boys and Men 16 through 26 Years of Age
The primary analyses of efficacy were conducted in the per-protocol efficacy (PPE) population. This population consisted of boys and men who received all 3 vaccinations within 1 year of enrollment, did not have major deviations from the study protocol, and were naïve (PCR negative and seronegative) to the relevant HPV type(s) (Types 6, 11, 16, and 18) prior to dose 1 and through 1 month postdose 3 (Month 7). Efficacy was measured starting after the Month 7 visit.
GARDASIL was efficacious in reducing the incidence of genital warts related to vaccine HPV types 6 and 11 in those boys and men who were PCR negative and seronegative at baseline (Table 12). Efficacy against penile/perineal/perianal intraepithelial neoplasia (PIN) grades 1/2/3 or penile/perineal/perianal cancer was not demonstrated as the number of cases was too limited to reach statistical significance.
Table 12: Analysis of Efficacy of GARDASIL in the PPE* Population of 16- Through 26-Year-Old Boys and Men for Vaccine HPV Types Endpoint GARDASIL AAHS Control % Efficacy (95% CI) N† Number of cases N Number of cases CI = Confidence Interval AAHS Control = Amorphous Aluminum Hydroxyphosphate Sulfate - *
- The PPE population consisted of individuals who received all 3 vaccinations within 1 year of enrollment, did not have major deviations from the study protocol, and were naïve (PCR negative and seronegative) to the relevant HPV type(s) (Types 6, 11, 16, and 18) prior to dose 1 and through 1 month postdose 3 (Month 7).
- †
- N = Number of individuals with at least 1 follow-up visit after Month 7
External Genital Lesions HPV 6-, 11-, 16-, or 18- related External Genital Lesions 1394 3 1404 32 90.6 (70.1, 98.2) Condyloma 1394 3 1404 28 89.3 (65.3, 97.9) PIN 1/2/3 1394 0 1404 4 100.0 (-52.1, 100.0) 14.3 Prophylactic Efficacy – Anal Disease Caused by HPV Types 6, 11, 16, and 18 in Boys and Men 16 through 26 Years of Age in the MSM Sub-study
A sub-study of Study 5 evaluated the efficacy of GARDASIL against anal disease (anal intraepithelial neoplasia and anal cancer) in a population of 598 MSM. The primary analyses of efficacy were conducted in the per-protocol efficacy (PPE) population of Study 5.
GARDASIL was efficacious in reducing the incidence of anal intraepithelial neoplasia (AIN) grades 1 (both condyloma and non-acuminate), 2, and 3 related to vaccine HPV types 6, 11, 16, and 18 in those boys and men who were PCR negative and seronegative at baseline (Table 13).
Table 13: Analysis of Efficacy of GARDASIL for Anal Disease in the PPE* Population of 16- Through 26-Year-Old Boys and Men in the MSM Sub-study for Vaccine HPV Types HPV 6-, 11-, 16-, or 18- related Endpoint GARDASIL AAHS Control % Efficacy (95% CI) N† Number of cases N Number of cases CI = Confidence Interval AAHS Control = Amorphous Aluminum Hydroxyphosphate Sulfate - *
- The PPE population consisted of individuals who received all 3 vaccinations within 1 year of enrollment, did not have major deviations from the study protocol, and were naïve (PCR negative and seronegative) to the relevant HPV type(s) (Types 6, 11, 16, and 18) prior to dose 1 and through 1 month postdose 3 (month 7).
- †
- N = Number of individuals with at least 1 follow-up visit after Month 7
AIN 1/2/3 194 5 208 24 77.5 (39.6, 93.3) AIN 2/3 194 3 208 13 74.9 (8.8, 95.4) AIN 1 194 4 208 16 73.0 (16.3, 93.4) Condyloma Acuminatum 194 0 208 6 100.0 (8.2, 100.0) Non-acuminate 194 4 208 11 60.4 (-33.5, 90.8) 14.4 Population Impact in Girls and Women 16 through 26 Years of Age
Effectiveness of GARDASIL in Prevention of HPV Types 6-, 11-, 16-, or 18-Related Genital Disease in Girls and Women 16 Through 26 Years of Age, Regardless of Current or Prior Exposure to Vaccine HPV Types
The clinical trials included girls and women regardless of current or prior exposure to vaccine HPV types, and additional analyses were conducted to evaluate the impact of GARDASIL with respect to HPV 6-, 11-, 16-, and 18-related cervical and genital disease in these girls and women. Here, analyses included events arising among girls and women regardless of baseline PCR status and serostatus, including HPV infections that were present at the start of vaccination as well as events that arose from infections that were acquired after the start of vaccination.
The impact of GARDASIL in girls and women regardless of current or prior exposure to a vaccine HPV type is shown in Table 14. Impact was measured starting 1 month Postdose 1. Prophylactic efficacy denotes the vaccine's efficacy in girls and women who are naïve (PCR negative and seronegative) to the relevant HPV types at Day 1. Vaccine impact in girls and women who were positive for vaccine HPV infection, as well as vaccine impact among girls and women regardless of baseline vaccine HPV PCR status and serostatus are also presented. The majority of CIN and genital warts, VIN, and VaIN related to a vaccine HPV type detected in the group that received GARDASIL occurred as a consequence of HPV infection with the relevant HPV type that was already present at Day 1.
There was no clear evidence of protection from disease caused by HPV types for which girls and women were PCR positive regardless of serostatus at baseline.
Table 14: Effectiveness of GARDASIL in Prevention of HPV 6, 11, 16, or 18-Related Genital Disease in Girls and Women 16 Through 26 Years of Age, Regardless of Current or Prior Exposure to Vaccine HPV Types Endpoint Analysis GARDASIL or HPV 16 L1 VLP Vaccine AAHS Control % Reduction
(95% CI)N Cases N Cases CI = Confidence Interval N = Number of individuals who have at least one follow-up visit after Day 1 Note 1: The 16- and 18-related CIN 2/3 or AIS composite endpoint included data from studies 1, 2, 3, and 4. All other endpoints only included data from studies 2, 3, and 4. Note 2: Positive status at Day 1 denotes PCR positive and/or seropositive for the respective type at Day 1. Note 3: Table 14 does not include disease due to non-vaccine HPV types. AAHS Control = Amorphous Aluminum Hydroxyphosphate Sulfate - *
- Includes all individuals who received at least 1 vaccination and who were HPV-naïve (i.e., seronegative and PCR negative) at Day 1 to the vaccine HPV type being analyzed. Case counting started at 1 month postdose 1.
- †
- Includes all individuals who received at least 1 vaccination and who were HPV positive or had unknown HPV status at Day 1, to at least one vaccine HPV type. Case counting started at Day 1.
- ‡
- Out of the 148 AAHS control cases of 16/18 CIN 2/3, 2 women were missing serology or PCR results for Day 1.
- §
- There is no expected efficacy since GARDASIL has not been demonstrated to provide protection against disease from vaccine HPV types to which a person has previously been exposed through sexual activity.
- ¶
- Includes all individuals who received at least 1 vaccination (regardless of baseline HPV status at Day 1). Case counting started at 1 month postdose 1.
- #
- Includes 2 AAHS control women with missing serology/PCR data at Day 1.
- Þ
- Includes 1 woman with missing serology/PCR data at Day 1.
HPV 16- or 18-related CIN 2/3 or AIS Prophylactic Efficacy* 9346 4 9407 155 97.4 (93.3, 99.3) HPV 16 and/or HPV 18 Positive at Day 1† 2870 142 2898 148‡ --§ Girls and Women Regardless of Current or Prior Exposure to HPV 16 or 18¶ 9836 146 9904 303 51.8 (41.1, 60.7) HPV 16- or 18-related VIN 2/3 or VaIN 2/3 Prophylactic Efficacy* 8642 1 8673 34 97.0 (82.4, 99.9) HPV 16 and/or HPV 18 Positive at Day 1† 1880 8 1876 4 --§ Girls and Women Regardless of Current or Prior Exposure to HPV 16 or 18¶ 8955 9 8968 38 76.3 (50.0, 89.9) HPV 6-, 11-, 16-, 18-related CIN (CIN 1, CIN 2/3) or AIS Prophylactic Efficacy* 8630 16 8680 309 94.8 (91.5, 97.1) HPV 6, HPV 11, HPV 16, and/or HPV 18 Positive at Day 1† 2466 186# 2437 213# --§ Girls and Women Regardless of Current or Prior Exposure to Vaccine HPV Types¶ 8819 202 8854 522 61.5 (54.6, 67.4) HPV 6-, 11-, 16-, or 18-related Genital Warts Prophylactic Efficacy* 8761 10 8792 252 96.0 (92.6, 98.1) HPV 6, HPV 11, HPV 16, and/or HPV 18 Positive at Day 1† 2501 51Þ 2475 55 Þ --§ Girls and Women Regardless of Current or Prior Exposure to Vaccine HPV Types¶ 8955 61 8968 307 80.3 (73.9, 85.3) HPV 6- or 11-related Genital Warts Prophylactic Efficacy* 7769 9 7792 246 96.4 (93.0, 98.4) HPV 6 and/or HPV 11 Positive at Day 1† 1186 51 1176 54 --§ Girls and Women Regardless of Current or Prior Exposure to Vaccine HPV Types¶ 8955 60 8968 300 80.1 (73.7, 85.2) Effectiveness of GARDASIL in Prevention of Any HPV Type Related Genital Disease in Girls and Women 16 Through 26 Years of Age, Regardless of Current or Prior Infection with Vaccine or Non-Vaccine HPV Types
The impact of GARDASIL against the overall burden of dysplastic or papillomatous cervical, vulvar, and vaginal disease regardless of HPV detection, results from a combination of prophylactic efficacy against vaccine HPV types, disease contribution from vaccine HPV types present at time of vaccination, the disease contribution from HPV types not contained in the vaccine, and disease in which HPV was not detected.
Additional efficacy analyses were conducted in 2 populations: (1) a generally HPV-naïve population (negative to 14 common HPV types and had a Pap test that was negative for SIL [Squamous Intraepithelial Lesion] at Day 1), approximating a population of sexually-naïve girls and women and (2) the general study population of girls and women regardless of baseline HPV status, some of whom had HPV-related disease at Day 1.
Among generally HPV-naïve girls and women and among all girls and women in the study population (including girls and women with HPV infection at Day 1), GARDASIL reduced the overall incidence of CIN 2/3 or AIS; of VIN 2/3 or VaIN 2/3; of CIN (any grade) or AIS; and of Genital Warts (Table 15). These reductions were primarily due to reductions in lesions caused by HPV types 6, 11, 16, and 18 in girls and women naïve (seronegative and PCR negative) for the specific relevant vaccine HPV type. Infected girls and women may already have CIN 2/3 or AIS at Day 1 and some will develop CIN 2/3 or AIS during follow-up, either related to a vaccine or non-vaccine HPV type present at the time of vaccination or related to a non-vaccine HPV type not present at the time of vaccination.
Table 15: Effectiveness of GARDASIL in Prevention of Any HPV Type Related Genital Disease in Girls and Women 16 Through 26 Years of Age, Regardless of Current or Prior Infection with Vaccine or Non-Vaccine HPV Types Endpoints Caused by Vaccine or Non-vaccine HPV Types Analysis GARDASIL AAHS Control % Reduction
(95% CI)N Cases N Cases CI = Confidence Interval AAHS Control = Amorphous Aluminum Hydroxyphosphate Sulfate - *
- Includes all individuals who received at least 1 vaccination and who had a Pap test that was negative for SIL [Squamous Intraepithelial Lesion] at Day 1 and were naïve to 14 common HPV types at Day 1. Case counting started at 1 month postdose 1.
- †
- Includes all individuals who received at least 1 vaccination (regardless of baseline HPV status or Pap test result at Day 1). Case counting started at 1 month postdose 1.
CIN 2/3 or AIS Prophylactic Efficacy* 4616 77 4680 136 42.7 (23.7, 57.3) Girls and Women Regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types† 8559 421 8592 516 18.4 (7.0, 28.4) VIN 2/3 and VaIN 2/3 Prophylactic Efficacy* 4688 7 4735 31 77.1 (47.1, 91.5) Girls and Women Regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types† 8688 30 8701 61 50.7 (22.5, 69.3) CIN (Any Grade) or AIS Prophylactic Efficacy* 4616 272 4680 390 29.7 (17.7, 40.0) Girls and Women Regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types† 8559 967 8592 1189 19.1 (11.9, 25.8) Genital Warts Prophylactic Efficacy* 4688 29 4735 169 82.8 (74.3, 88.8) Girls and Women Regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types† 8688 132 8701 350 62.5 (54.0, 69.5) 14.5 Population Impact in Boys and Men 16 through 26 Years of Age
Effectiveness of GARDASIL in Prevention of HPV Types 6-, 11-, 16-, or 18-Related Anogenital Disease in Boys and Men 16 Through 26 Years of Age, Regardless of Current or Prior Exposure to Vaccine HPV Types
Study 5 included boys and men regardless of current or prior exposure to vaccine HPV types, and additional analyses were conducted to evaluate the impact of GARDASIL with respect to HPV 6-, 11-, 16-, and 18-related anogenital disease in these boys and men. Here, analyses included events arising among boys and men regardless of baseline PCR status and serostatus, including HPV infections that were present at the start of vaccination as well as events that arose from infections that were acquired after the start of vaccination.
The impact of GARDASIL in boys and men regardless of current or prior exposure to a vaccine HPV type is shown in Table 16. Impact was measured starting at Day 1. Prophylactic efficacy denotes the vaccine's efficacy in boys and men who are naïve (PCR negative and seronegative) to the relevant HPV types at Day 1. Vaccine impact in boys and men who were positive for vaccine HPV infection, as well as vaccine impact among boys and men regardless of baseline vaccine HPV PCR status and serostatus are also presented. The majority of anogenital disease related to a vaccine HPV type detected in the group that received GARDASIL occurred as a consequence of HPV infection with the relevant HPV type that was already present at Day 1.
There was no clear evidence of protection from disease caused by HPV types for which boys and men were PCR positive regardless of serostatus at baseline.
Table 16: Effectiveness of GARDASIL in Prevention of HPV Types 6-, 11-, 16-, or 18-Related Anogenital Disease in Boys and Men 16 Through 26 Years of Age, Regardless of Current or Prior Exposure to Vaccine HPV Types Endpoint Analysis GARDASIL AAHS Control % Reduction
(95% CI)N Cases N Cases CI = Confidence Interval AAHS Control = Amorphous Aluminum Hydroxyphosphate Sulfate - *
- Includes all individuals who received at least 1 vaccination and who were HPV-naïve (i.e., seronegative and PCR negative) at Day 1 to the vaccine HPV type being analyzed. Case counting started at Day 1.
- †
- Includes all individuals who received at least 1 vaccination and who were HPV positive or had unknown HPV status at Day 1, to at least one vaccine HPV type. Case counting started at Day 1.
- ‡
- There is no expected efficacy since GARDASIL has not been demonstrated to provide protection against disease from vaccine HPV types to which a person has previously been exposed through sexual activity.
- §
- Includes all individuals who received at least 1 vaccination. Case counting started at Day 1.
External Genital Lesions Prophylactic Efficacy* 1775 13 1770 54 76.3 (56.0, 88.1) HPV 6, HPV 11, HPV 16, and/or HPV 18 Positive at Day 1† 460 14 453 26 --‡ Boys and Men Regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types§ 1943 27 1937 80 66.7 (48.0, 79.3) Condyloma Prophylactic Efficacy* 1775 10 1770 49 80.0 (59.9, 90.9) HPV 6, HPV 11, HPV 16, and/or HPV 18 Positive at Day 1† 460 14 453 25 --‡ Boys and Men Regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types§ 1943 24 1937 74 68.1 (48.8, 80.7) PIN 1/2/3 Prophylactic Efficacy* 1775 4 1770 5 20.7 (-268.4, 84.3) HPV 6, HPV 11, HPV 16, and/or HPV 18 Positive at Day 1† 460 2 453 1 --‡ Boys and Men Regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types§ 1943 6 1937 6 0.3 (-272.8, 73.4) AIN 1/2/3 Prophylactic Efficacy* 259 9 261 39 76.9 (51.4, 90.1) HPV 6, HPV 11, HPV 16, and/or HPV 18 Positive at Day 1† 103 29 116 38 --‡ Boys and Men Regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types§ 275 38 276 77 50.3 (25.7, 67.2) AIN 2/3 Prophylactic Efficacy* 259 7 261 19 62.5 (6.9, 86.7) HPV 6, HPV 11, HPV 16, and/or HPV 18 Positive at Day 1† 103 11 116 20 --‡ Boys and Men Regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types§ 275 18 276 39 54.2 (18.0, 75.3) Effectiveness of GARDASIL in Prevention of Any HPV Type Related Anogenital Disease in Boys and Men 16 Through 26 Years of Age, Regardless of Current or Prior Infection with Vaccine or Non-Vaccine HPV Types
The impact of GARDASIL against the overall burden of dysplastic or papillomatous anogenital disease regardless of HPV detection, results from a combination of prophylactic efficacy against vaccine HPV types, disease contribution from vaccine HPV types present at time of vaccination, the disease contribution from HPV types not contained in the vaccine, and disease in which HPV was not detected.
Additional efficacy analyses from Study 5 were conducted in 2 populations: (1) a generally HPV-naïve population that consisted of boys and men who are seronegative and PCR negative to HPV 6, 11, 16, and 18 and PCR negative to HPV 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59 at Day 1, approximating a population of sexually-naïve boys and men and (2) the general study population of boys and men regardless of baseline HPV status, some of whom had HPV-related disease at Day 1.
Among generally HPV-naïve boys and men and among all boys and men in Study 5 (including boys and men with HPV infection at Day 1), GARDASIL reduced the overall incidence of anogenital disease (Table 17). These reductions were primarily due to reductions in lesions caused by HPV types 6, 11, 16, and 18 in boys and men naïve (seronegative and PCR negative) for the specific relevant vaccine HPV type. Infected boys and men may already have anogenital disease at Day 1 and some will develop anogenital disease during follow-up, either related to a vaccine or non-vaccine HPV type present at the time of vaccination or related to a non-vaccine HPV type not present at the time of vaccination.
Table 17: Effectiveness of GARDASIL in Prevention of Any HPV Type Related Anogenital Disease in Boys and Men 16 Through 26 Years of Age, Regardless of Current or Prior Infection with Vaccine or Non-Vaccine HPV Types Endpoint Analysis GARDASIL AAHS Control % Reduction
(95% CI)N Cases N Cases CI = Confidence Interval AAHS Control = Amorphous Aluminum Hydroxyphosphate Sulfate - *
- Includes all individuals who received at least 1 vaccination and who were seronegative and PCR negative at enrollment to HPV 6, 11, 16 and 18, and PCR negative at enrollment to HPV 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59. Case counting started at Day 1.
- †
- Includes all individuals who received at least 1 vaccination. Case counting started at Day 1.
External Genital Lesions Prophylactic Efficacy* 1275 7 1270 37 81.5 (58.0, 93.0) Boys and Men Regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types† 1943 38 1937 92 59.3 (40.0, 72.9) Condyloma Prophylactic Efficacy* 1275 5 1270 33 85.2 (61.8, 95.5) Boys and Men Regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types† 1943 33 1937 85 61.8 (42.3, 75.3) PIN 1/2/3 Prophylactic Efficacy* 1275 2 1270 4 50.7 (-244.3, 95.5) Boys and Men Regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types† 1943 8 1937 7 -13.9 (-269.0, 63.9) AIN 1/2/3 Prophylactic Efficacy* 129 12 126 28 54.9 (8.4, 79.1) Boys and Men Regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types† 275 74 276 103 25.7 (-1.1, 45.6) AIN 2/3 Prophylactic Efficacy* 129 8 126 18 52.5 (-14.8, 82.1) Boys and Men Regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types† 275 44 276 59 24.3 (-13.8, 50.0) 14.6 Overall Population Impact
The subject characteristics (e.g. lifetime sex partners, geographic distribution of the subjects) influence the HPV prevalence of the population and therefore the population benefit can vary widely.
The overall efficacy of GARDASIL will vary with the baseline prevalence of HPV infection and disease, the incidence of infections against which GARDASIL has shown protection, and those infections against which GARDASIL has not been shown to protect.
The efficacy of GARDASIL for HPV types not included in the vaccine (i.e., cross-protective efficacy) is a component of the overall impact of the vaccine on rates of disease caused by HPV. Cross-protective efficacy was not demonstrated against disease caused by non-vaccine HPV types in the combined database of the Study 3 and Study 4 trials.
GARDASIL does not protect against genital disease not related to HPV. One woman who received GARDASIL in Study 3 developed an external genital well-differentiated squamous cell carcinoma at Month 24. No HPV DNA was detected in the lesion or in any other samples taken throughout the study.
In 18,150 girls and women enrolled in Study 2, Study 3, and Study 4, GARDASIL reduced definitive cervical therapy procedures by 23.9% (95% CI: 15.2%, 31.7%).
14.7 Studies in Women 27 through 45 Years of Age
Study 6 evaluated efficacy in 3253 women 27 through 45 years of age based on a combined endpoint of HPV 6-, 11-, 16- or 18-related persistent infection, genital warts, vulvar and vaginal dysplastic lesions of any grade, CIN of any grade, AIS, and cervical cancer. These women were randomized 1:1 to receive either GARDASIL or AAHS control. The efficacy for the combined endpoint was driven primarily by prevention of persistent infection. There was no statistically significant efficacy demonstrated for CIN 2/3, AIS, or cervical cancer. In post hoc analyses conducted to assess the impact of GARDASIL on the individual components of the combined endpoint, the results in the population of women naïve to the relevant HPV type at baseline were as follows: prevention of HPV 6-, 11-, 16- or 18-related persistent infection (80.5% [95% CI: 68.3, 88.6]), prevention of HPV 6-, 11-, 16- or 18-related CIN (any grade) (85.8% [95% CI: 52.4, 97.3]), and prevention of HPV 6-, 11-, 16- or 18-related genital warts (87.6% [95% CI: 7.3, 99.7]).
Efficacy for disease endpoints was diminished in a population impact assessment of women who were vaccinated regardless of baseline HPV status (full analysis set). In the full analysis set (FAS), efficacy was not demonstrated for the following endpoints: prevention of HPV 16- and 18-related CIN 2/3, AIS, or cervical cancer and prevention of HPV 6- and 11-related condyloma. No efficacy was demonstrated against CIN 2/3, AIS, or cervical cancer in the general population irrespective of HPV type (FAS any type analysis).
14.8 Immunogenicity
Assays to Measure Immune Response
The minimum anti-HPV titer that confers protective efficacy has not been determined.
Because there were few disease cases in individuals naïve (PCR negative and seronegative) to vaccine HPV types at baseline in the group that received GARDASIL, it has not been possible to establish minimum anti-HPV 6, anti-HPV 11, anti-HPV 16, and anti-HPV 18 antibody levels that protect against clinical disease caused by HPV 6, 11, 16, and/or 18.
The immunogenicity of GARDASIL was assessed in 23,951 9- through 45-year-old girls and women (GARDASIL N = 12,634; AAHS control or saline placebo N = 11,317) and 5417 9- through 26-year-old boys and men (GARDASIL N = 3109; AAHS control or saline placebo N = 2308).
Type-specific immunoassays with type-specific standards were used to assess immunogenicity to each vaccine HPV type. These assays measured antibodies against neutralizing epitopes for each HPV type. The scales for these assays are unique to each HPV type; thus, comparisons across types and to other assays are not appropriate.
Immune Response to GARDASIL
The primary immunogenicity analyses were conducted in a per-protocol immunogenicity (PPI) population. This population consisted of individuals who were seronegative and PCR negative to the relevant HPV type(s) at enrollment, remained HPV PCR negative to the relevant HPV type(s) through 1 month postdose 3 (Month 7), received all 3 vaccinations, and did not deviate from the study protocol in ways that could interfere with the effects of the vaccine.
Immunogenicity was measured by (1) the percentage of individuals who were seropositive for antibodies against the relevant vaccine HPV type, and (2) the Geometric Mean Titer (GMT).
In clinical studies in 16- through 26-year-old girls and women, 99.8%, 99.8%, 99.8%, and 99.4% who received GARDASIL became anti-HPV 6, anti-HPV 11, anti-HPV 16, and anti-HPV 18 seropositive, respectively, by 1 month postdose 3 across all age groups tested.
In clinical studies in 27- through 45-year-old women, 98.2%, 97.9%, 98.6%, and 97.1% who received GARDASIL became anti-HPV 6, anti-HPV 11, anti-HPV 16, and anti-HPV 18 seropositive, respectively, by 1 month postdose 3 across all age groups tested.
In clinical studies in 16- through 26-year-old boys and men, 98.9%, 99.2%, 98.8%, and 97.4% who received GARDASIL became anti-HPV 6, anti-HPV 11, anti-HPV 16, and anti-HPV 18 seropositive, respectively, by 1 month postdose 3 across all age groups tested.
Across all populations, anti-HPV 6, anti-HPV 11, anti-HPV 16, and anti-HPV 18 GMTs peaked at Month 7 (Table 18 and Table 19). GMTs declined through Month 24 and then stabilized through Month 36 at levels above baseline. Tables 20 and 21 display the persistence of anti-HPV cLIA geometric mean titers by gender and age group. The duration of immunity following a complete schedule of immunization with GARDASIL has not been established.
Table 18: Summary of Month 7 Anti-HPV cLIA Geometric Mean Titers in the PPI* Population of Girls and Women Population N† n‡ % Seropositive
(95% CI)GMT
(95% CI) mMU§/mLcLIA = Competitive Luminex Immunoassay CI = Confidence Interval GMT = Geometric Mean Titers - *
- The PPI population consisted of individuals who received all 3 vaccinations within pre-defined day ranges, did not have major deviations from the study protocol, met predefined criteria for the interval between the Month 6 and Month 7 visit, and were naïve (PCR negative and seronegative) to the relevant HPV type(s) (types 6, 11, 16, and 18) prior to dose 1 and through 1 month Postdose 3 (Month 7).
- †
- Number of individuals randomized to the respective vaccination group who received at least 1 injection.
- ‡
- Number of individuals contributing to the analysis.
- §
- mMU = milli-Merck Units
Anti-HPV 6 9- through 15-year-old girls 1122 917 99.9 (99.4, 100.0) 929.2 (874.6, 987.3) 16- through 26-year-old girls and women 9859 3329 99.8 (99.6, 99.9) 545.0 (530.1, 560.4) 27- through 34-year-old women 667 439 98.4 (96.7, 99.4) 435.6 (393.4, 482.4) 35- through 45-year-old women 957 644 98.1 (96.8, 99.0) 397.3 (365.2, 432.2) Anti-HPV 11 9- through 15-year-old girls 1122 917 99.9 (99.4, 100.0) 1304.6 (1224.7, 1389.7) 16- through 26-year-old girls and women 9859 3353 99.8 (99.5, 99.9) 748.9 (726.0, 772.6) 27- through 34-year-old women 667 439 98.2 (96.4, 99.2) 577.9 (523.8, 637.5) 35- through 45-year-old women 957 644 97.7 (96.2, 98.7) 512.8 (472.9, 556.1) Anti-HPV 16 9- through 15-year-old girls 1122 915 99.9 (99.4, 100.0) 4918.5 (4556.6, 5309.1) 16- through 26-year-old girls and women 9859 3249 99.8 (99.6, 100.0) 2409.2 (2309.0, 2513.8) 27- through 34-year-old women 667 435 99.3 (98.0, 99.9) 2342.5 (2119.1, 2589.6) 35- through 45-year-old women 957 657 98.2 (96.8, 99.1) 2129.5 (1962.7, 2310.5) Anti-HPV 18 9- through 15-year-old girls 1122 922 99.8 (99.2, 100.0) 1042.6 (967.6, 1123.3) 16- through 26-year-old girls and women 9859 3566 99.4 (99.1, 99.7) 475.2 (458.8, 492.1) 27- through 34-year-old women 667 501 98.0 (96.4, 99.0) 385.8 (347.6, 428.1) 35- through 45-year-old women 957 722 96.4 (94.8, 97.6) 324.6 (297.6, 354.0) Table 19: Summary of Month 7 Anti-HPV cLIA Geometric Mean Titers in the PPI* Population of Boys and Men Population N† n‡ % Seropositive
(95% CI)GMT
(95% CI) mMU§/mLcLIA = Competitive Luminex Immunoassay CI = Confidence Interval GMT = Geometric Mean Titers - *
- The PPI population consisted of individuals who received all 3 vaccinations within pre-defined day ranges, did not have major deviations from the study protocol, met predefined criteria for the interval between the Month 6 and Month 7 visit, and were naïve (PCR negative and seronegative) to the relevant HPV type(s) (types 6, 11, 16, and 18) prior to dose 1 and through 1 month Postdose 3 (Month 7).
- †
- Number of individuals randomized to the respective vaccination group who received at least 1 injection.
- ‡
- Number of individuals contributing to the analysis.
- §
- mMU = milli-Merck Units
Anti-HPV 6 9- through 15-year-old boys 1072 884 99.9 (99.4, 100.0) 1037.5 (963.5, 1117.3) 16- through 26-year-old boys and men 2026 1093 98.9 (98.1, 99.4) 447.8 (418.9, 478.6) Anti-HPV 11 9- through 15-year-old boys 1072 885 99.9 (99.4, 100.0) 1386.8 (1298.5, 1481.0) 16- through 26-year-old boys and men 2026 1093 99.2 (98.4, 99.6) 624.3 (588.4, 662.3) Anti-HPV 16 9- through 15-year-old boys 1072 882 99.8 (99.2, 100.0) 6056.5 (5601.3, 6548.7) 16- through 26-year-old boys and men 2026 1136 98.8 (97.9, 99.3) 2403.3 (2243.4, 2574.6) Anti-HPV 18 9- through 15-year-old boys 1072 887 99.8 (99.2, 100) 1357.4 (1249.4, 1474.7) 16- through 26-year-old boys and men 2026 1175 97.4 (96.3, 98.2) 402.6 (374.6, 432.7) Table 20: Persistence of Anti-HPV cLIA Geometric Mean Titers in 9- Through 45-Year-Old Girls and Women Assay (cLIA)/ Time Point 9- to 15-Year-Old Girls
(N* = 1122)16- to 26-Year-Old Girls and Women
(N* = 9859)27- to 34-Year-Old Women
(N* = 667)35- to 45-Year-Old Women
(N* = 957)n† GMT
(95% CI) mMU‡/mLn† GMT
(95% CI) mMU‡/mLn† GMT
(95% CI) mMU‡/mLn† GMT
(95% CI) mMU‡/mLcLIA = Competitive Luminex Immunoassay CI = Confidence Interval GMT = Geometric Mean Titers - *
- N = Number of individuals randomized in the respective group who received at least 1 injection.
- †
- n = Number of individuals in the indicated immunogenicity population.
- ‡
- mMU = milli-Merck Units
- §
- Month 37 for 9- to 15-year-old girls. No serology samples were collected at this time point for 16- to 26-year-old girls and women.
- ¶
- Month 48/End-of-study visits for 16- to 26-year-old girls and women were generally scheduled earlier than Month 48. Mean visit timing was Month 44. The studies in 9- to 15-year-old girls were planned to end prior to 48 months and therefore no serology samples were collected.
Anti-HPV 6 Month 07 917 929.2
(874.6, 987.3)3329 545.0
(530.1, 560.4)439 435.6
(393.4, 482.4)644 397.3
(365.2, 432.2)Month 24 214 156.1
(135.6, 179.6)2788 109.1
(105.2, 113.1)421 70.7
(63.8, 78.5)628 69.3
(63.7, 75.4)Month 36§ 356 129.4
(115.6, 144.8)- - 399 79.5
(72.0, 87.7)618 81.1
(75.0, 87.8)Month 48¶ - - 2514 73.8
(70.9, 76.8)391 58.8
(52.9, 65.3)616 62.0
(57.0, 67.5)Anti-HPV 11 Month 07 917 1304.6
(1224.7, 1389.7)3353 748.9
(726.0, 772.6)439 577.9
(523.8, 637.5)644 512.8
(472.9, 556.1)Month 24 214 218.0
(188.3, 252.4)2817 137.1
(132.1, 142.3)421 79.3
(71.5, 87.8)628 73.4
(67.4, 79.8)Month 36§ 356 148.0
(131.1, 167.1)- - 399 81.8
(74.3, 90.1)618 77.4
(71.6, 83.6)Month 48¶ - - 2538 89.4
(85.9, 93.1)391 67.4
(60.9, 74.7)616 62.7
(57.8, 68.0)Anti-HPV 16 Month 07 915 4918.5
(4556.6, 5309.1)3249 2409.2
(2309.0, 2513.8)435 2342.5
(2119.1, 2589.6)657 2129.5
(1962.7, 2310.5)Month 24 211 944.2
(804.4, 1108.3)2721 442.6
(425.0, 460.9)416 285.9
(254.4, 321.2)642 271.4
(247.1, 298.1)Month 36§ 353 642.2
(562.8, 732.8)- - 399 291.5
(262.5, 323.8)631 276.7
(254.5, 300.8)Month 48¶ - - 2474 326.2
(311.8, 341.3)394 211.8
(189.5, 236.8)628 192.8
(176.5, 210.6)Anti-HPV 18 Month 07 922 1042.6
(967.6, 1123.3)3566 475.2
(458.8, 492.1)501 385.8
(347.6, 428.1)722 324.6
(297.6, 354.0)Month 24 214 137.7
(114.8, 165.1)3002 50.8
(48.2, 53.5)478 31.8
(28.1, 36.0)705 26.0
(23.5, 28.8)Month 36§ 357 87.0
(74.8, 101.2)- - 453 32.1
(28.5, 36.3)689 27.0
(24.5, 29.8)Month 48¶ - - 2710 33.2
(31.5, 35.0)444 25.2
(22.3, 28.5)688 21.2
(19.2, 23.4)Table 21: Persistence of Anti-HPV cLIA Geometric Mean Titers in 9- Through 26-Year-Old Boys and Men Assay (cLIA)/ Time Point 9- to 15-Year-Old Boys
(N* = 1072)16- to 26-Year-Old Boys and Men
(N* = 2026)n† GMT
(95% CI) mMU‡/mLn† GMT
(95% CI) mMU‡/mLcLIA = Competitive Luminex Immunoassay CI = Confidence Interval GMT = Geometric Mean Titers - *
- N = Number of individuals randomized in the respective group who received at least 1 injection.
- †
- n = Number of individuals in the indicated immunogenicity population.
- ‡
- mMU = milli-Merck Units
- §
- Month 36 time point for 16- to 26-year-old boys and men; Month 37 for 9- to 15-year-old boys.
- ¶
- The studies in 9- to 15-year-old boys and girls and 16- to 26-year-old boys and men were planned to end prior to 48 months and therefore no serology samples were collected.
Anti-HPV 6 Month 07 884 1037.5
(963.5, 1117.3)1094 447.2
(418.4, 477.9)Month 24 323 134.1
(119.5, 150.5)907 80.3
(74.9, 86.0)Month 36§ 342 126.6
(111.9, 143.2)654 72.4
(68.0, 77.2)Month 48¶ - - - - Anti-HPV 11 Month 07 885 1386.8
(1298.5, 1481.0)1094 624.5
(588.6, 662.5)Month 24 324 188.5
(168.4, 211.1)907 94.6
(88.4, 101.2)Month 36§ 342 148.8
(131.1, 169.0)654 80.3
(75.7, 85.2)Month 48¶ - - - - Anti-HPV 16 Month 07 882 6056.5
(5601.4, 6548.6)1137 2401.5
(2241.8, 2572.6)Month 24 322 938.2
(825.0, 1067.0)938 347.7
(322.5, 374.9)Month 36§ 341 708.8
(613.9, 818.3)672 306.7
(287.5, 327.1)Month 48¶ - - - - Anti-HPV 18 Month 07 887 1357.4
(1249.4, 1474.7)1176 402.6
(374.6, 432.6)Month 24 324 131.9
(112.1, 155.3)967 38.7
(35.2, 42.5)Month 36§ 343 113.0
(94.7, 135.0)690 33.4
(30.9, 36.1)Month 48¶ - - - - Tables 18 and 19 display the Month 7 immunogenicity data for girls and women and boys and men. Anti-HPV responses 1 month postdose 3 among 9- through 15-year-old adolescent girls were non-inferior to anti-HPV responses in 16- through 26-year-old girls and women in the combined database of immunogenicity studies for GARDASIL. Anti-HPV responses 1 month postdose 3 among 9- through 15-year-old adolescent boys were non-inferior to anti-HPV responses in 16- through 26-year-old boys and men in Study 5.
On the basis of this immunogenicity bridging, the efficacy of GARDASIL in 9- through 15-year-old adolescent girls and boys is inferred.
GMT Response to Variation in Dosing Regimen in 18- Through 26-Year-Old Women
Girls and women evaluated in the PPE population of clinical studies received all 3 vaccinations within 1 year of enrollment. An analysis of immune response data suggests that flexibility of ±1 month for Dose 2 (i.e., Month 1 to Month 3 in the vaccination regimen) and flexibility of ±2 months for Dose 3 (i.e., Month 4 to Month 8 in the vaccination regimen) do not impact the immune responses to GARDASIL.
Duration of the Immune Response to GARDASIL
The duration of immunity following a complete schedule of immunization with GARDASIL has not been established. The peak anti-HPV GMTs for HPV types 6, 11, 16, and 18 occurred at Month 7. Anti-HPV GMTs for HPV types 6, 11, 16, and 18 were similar between measurements at Month 24 and Month 60 in Study 2.
14.9 Long-Term Follow-Up Studies
The protection of GARDASIL against HPV-related disease continues to be studied over time in populations including adolescents (boys and girls) and women who were enrolled in the Phase 3 studies.
Persistence of Effectiveness
An extension of Study 4 used national healthcare registries in Denmark, Iceland, Norway, and Sweden to monitor endpoint cases of HPV 6-, 11-, 16-, or 18-related CIN (any grade), AIS, cervical cancer, vulvar cancer, or vaginal cancer among 2,650 girls and women 16 through 23 years of age at enrollment who were randomized to vaccination with GARDASIL and consented to be followed in the extension study. An interim analysis of the per-protocol effectiveness population included 1,902 subjects who completed the GARDASIL vaccination series within one year, were naïve to the relevant HPV type through 1 month postdose 3, had no protocol violations, and had follow-up data available. The median follow-up from initial vaccination was 6.7 years with a range of 2.8 to 8.4 years. No cases of HPV 6-, 11-, 16-, or 18-related CIN (any grade), AIS, cervical cancer, vulvar cancer, or vaginal cancer were observed over a total of 5,765 person-years at risk.
An extension of a Phase 3 study (Study 7) in which 614 girls and 565 boys 9 through 15 years of age at enrollment were randomized to vaccination with GARDASIL actively followed subjects for endpoint cases of HPV 6-, 11-, 16-, or 18-related persistent infection, CIN (any grade), AIS, VIN, VaIN, cervical cancer, vulvar cancer, vaginal cancer, and genital lesions from the initiation of sexual activity or age 16 onwards. An interim analysis of the per-protocol effectiveness population included 246 girls and 168 boys who completed the GARDASIL vaccination series within one year, were seronegative to the relevant HPV type at initiation of the vaccination series, and had not initiated sexual activity prior to receiving the third dose of GARDASIL. The median follow-up, from the first dose of vaccine, was 7.2 years with a range of 0.5 to 8.5 years. No cases of persistent infection of at least 12 months' duration and no cases of HPV 6-, 11-, 16-, or 18-related CIN (any grade), AIS, VIN, VaIN, cervical cancer, vulvar cancer, vaginal cancer, or genital lesions were observed over a total 1,105 person-years at risk. There were 4 cases of HPV 6-, 11-, 16-, or 18-related persistent infection of at least 6 months' duration, including 3 cases related to HPV 16 and 1 case related to HPV 6, none of which persisted to 12 months' duration.
Persistence of the Immune Response
The interim reports of the two extension studies described above included analyses of type-specific anti-HPV antibody titers at 9 years postdose 1 for girls and women 16 through 23 years of age at enrollment (range of 1,178 to 1,331 subjects with evaluable data across HPV types) and at 8 years postdose 1 for boys and girls 9 through 15 years of age at enrollment (range of 436 to 440 subjects with evaluable data across HPV types). Anti-HPV 6, 11, 16, and 18 GMTs as measured by cLIA were decreased compared with corresponding values at earlier time points, but the proportions of seropositive subjects ranged from 88.4% to 94.4% for anti-HPV 6, from 89.1% to 95.5% for anti-HPV 11, from 96.8% to 99.1% for anti-HPV 16, and from 60.0% to 64.1% for anti-HPV 18.
14.10 Studies with RECOMBIVAX HB [hepatitis B vaccine (recombinant)]
The safety and immunogenicity of co-administration of GARDASIL with RECOMBIVAX HB [hepatitis B vaccine (recombinant)] (same visit, injections at separate sites) were evaluated in a randomized, double-blind, study of 1871 women aged 16 through 24 years at enrollment. The race distribution of the girls and women in the clinical trial was as follows: 61.6% White; 1.6% Hispanic (Black and White); 23.8% Other; 11.9% Black; 0.8% Asian; and 0.3% American Indian.
Subjects either received GARDASIL and RECOMBIVAX HB (n = 466), GARDASIL and RECOMBIVAX HB-matched placebo (n = 468), RECOMBIVAX HB and GARDASIL-matched placebo (n = 467) or RECOMBIVAX-matched placebo and GARDASIL-matched placebo (n = 470) at Day 1, Month 2 and Month 6. Immunogenicity was assessed for all vaccines 1 month post completion of the vaccination series.
Concomitant administration of GARDASIL with RECOMBIVAX HB [hepatitis B vaccine (recombinant)] did not interfere with the antibody response to any of the vaccine antigens when GARDASIL was given concomitantly with RECOMBIVAX HB or separately.
14.11 Studies with Menactra [Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine] and Adacel [Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap)]
The safety and immunogenicity of co-administration of GARDASIL with Menactra [Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine] and Adacel [Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap)] (same visit, injections at separate sites) were evaluated in an open-labeled, randomized, controlled study of 1040 boys and girls 11 through 17 years of age at enrollment. The race distribution of the subjects in the clinical trial was as follows: 77.7% White; 6.8% Hispanic (Black and White); 1.4% Multi-racial; 12.3% Black; 1.2% Asian; 0.2% Indian; and 0.4% American Indian.
One group received GARDASIL in one limb and both Menactra and Adacel, as separate injections, in the opposite limb concomitantly on Day 1 (n = 517). The second group received the first dose of GARDASIL on Day 1 in one limb then Menactra and Adacel, as separate injections, at Month 1 in the opposite limb (n = 523). Subjects in both vaccination groups received the second dose of GARDASIL at Month 2 and the third dose at Month 6. Immunogenicity was assessed for all vaccines 1 month post completion of the vaccination series (1 dose for Menactra and Adacel and 3 doses for GARDASIL).
Concomitant administration of GARDASIL with Menactra [Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine] and Adacel [Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap)] did not interfere with the antibody response to any of the vaccine antigens when GARDASIL was given concomitantly with Menactra and Adacel or separately.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
All presentations for GARDASIL contain a suspension of 120 mcg L1 protein from HPV types 6, 11, 16, and 18 in a 0.5-mL dose. GARDASIL is supplied in vials and syringes.
Carton of one 0.5-mL single-dose vial. NDC 0006-4045-00.
Carton of ten 0.5-mL single-dose vials. NDC 0006-4045-41.
Carton of six 0.5-mL single-dose prefilled Luer-Lok® syringes with tip caps. NDC 0006-4109-09.
Carton of ten 0.5-mL single-dose prefilled Luer-Lok® syringes with tip caps. NDC 0006-4109-02.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Inform the patient, parent, or guardian:
- Vaccination does not eliminate the necessity for women to continue to undergo recommended cervical cancer screening. Women who receive GARDASIL should continue to undergo cervical cancer screening per standard of care.
- Recipients of GARDASIL should not discontinue anal cancer screening if it has been recommended by a health care provider.
- GARDASIL has not been demonstrated to provide protection against disease from vaccine and non-vaccine HPV types to which a person has previously been exposed through sexual activity.
- Since syncope has been reported following vaccination sometimes resulting in falling with injury, observation for 15 minutes after administration is recommended.
- Vaccine information is required to be given with each vaccination to the patient, parent, or guardian.
- Information regarding benefits and risks associated with vaccination.
- GARDASIL is not recommended for use in pregnant women.
- Importance of completing the immunization series unless contraindicated.
- Report any adverse reactions to their health care provider.
-
SPL UNCLASSIFIED SECTION
Manuf. and Dist. by: Merck Sharp & Dohme LLC
Rahway, NJ 07065, USAFor patent information: www.msd.com/research/patent
The trademarks depicted herein are owned by their respective companies.
Copyright © 2006-2023 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.uspi-v501-i-2304r022
Printed in USA
-
PATIENT PACKAGE INSERT
USPPI
Patient Information about
GARDASIL® (pronounced "gard-Ah-sill")
Generic name: [Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant]Read this information with care before getting GARDASIL. You (the person getting GARDASIL) will need 3 doses of the vaccine. It is important to read this leaflet when you get each dose. This leaflet does not take the place of talking with your health care provider about GARDASIL.
What is GARDASIL?
GARDASIL is a vaccine (injection/shot) that is used for girls and women 9 through 26 years of age to help protect against the following diseases caused by Human Papillomavirus (HPV):
- Cervical cancer
- Vulvar and vaginal cancers
- Anal cancer
- Genital warts
- Precancerous cervical, vaginal, vulvar, and anal lesions
GARDASIL is used for boys and men 9 through 26 years of age to help protect against the following diseases caused by HPV:
- Anal cancer
- Genital warts
- Precancerous anal lesions
- The diseases listed above have many causes, and GARDASIL only protects against diseases caused by certain kinds of HPV (called Type 6, Type 11, Type 16, and Type 18). Most of the time, these 4 types of HPV are responsible for the diseases listed above.
- GARDASIL cannot protect you from a disease that is caused by other types of HPV, other viruses, or bacteria.
- GARDASIL does not treat HPV infection.
- You cannot get HPV or any of the above diseases from GARDASIL.
What important information about GARDASIL should I know?
- You should continue to get routine cervical cancer screening.
- GARDASIL may not fully protect everyone who gets the vaccine.
- GARDASIL will not protect against HPV types that you already have.
Who should not get GARDASIL?
You should not get GARDASIL if you have, or have had:
- an allergic reaction after getting a dose of GARDASIL.
- a severe allergic reaction to yeast, amorphous aluminum hydroxyphosphate sulfate, polysorbate 80.
What should I tell my health care provider before getting GARDASIL?
Tell your health care provider if you:
- are pregnant or planning to get pregnant. GARDASIL is not recommended for use in pregnant women.
- have immune problems, like HIV infection, cancer, or you take medicines that affect your immune system.
- have a fever over 100°F (37.8°C).
- had an allergic reaction to another dose of GARDASIL.
- take any medicines, even those you can buy over the counter.
Your health care provider will help decide if you should get the vaccine.
How is GARDASIL given?
GARDASIL is a shot that is usually given in the arm muscle. You will need 3 shots given on the following schedule:
- Dose 1: at a date you and your health care provider choose.
- Dose 2: 2 months after Dose 1.
- Dose 3: 6 months after Dose 1.
Fainting can happen after getting GARDASIL. Sometimes people who faint can fall and hurt themselves. For this reason, your health care provider may ask you to sit or lie down for 15 minutes after you get GARDASIL. Some people who faint might shake or become stiff. This may require evaluation or treatment by your health care provider.
Make sure that you get all 3 doses on time so that you get the best protection. If you miss a dose, talk to your health care provider.
Can other vaccines and medications be given at the same time as GARDASIL?
GARDASIL can be given at the same time as RECOMBIVAX HB®1 [hepatitis B vaccine (recombinant)] or Menactra [Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine] and Adacel [Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap)].
What are the possible side effects of GARDASIL?
The most common side effects with GARDASIL are:
- pain, swelling, itching, bruising, and redness at the injection site
- headache
- fever
- nausea
- dizziness
- vomiting
- fainting
There was no increase in side effects when GARDASIL was given at the same time as RECOMBIVAX HB [hepatitis B vaccine (recombinant)].
There was more injection-site swelling at the injection site for GARDASIL when GARDASIL was given at the same time as Menactra [Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine] and Adacel [Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap)].
Tell your health care provider if you have any of the following problems because these may be signs of an allergic reaction:
- difficulty breathing
- wheezing (bronchospasm)
- hives
- rash
Tell your health care provider if you have:
- swollen glands (neck, armpit, or groin)
- joint pain
- unusual tiredness, weakness, or confusion
- chills
- generally feeling unwell
- leg pain
- shortness of breath
- chest pain
- aching muscles
- muscle weakness
- seizure
- bad stomach ache
- bleeding or bruising more easily than normal
- skin infection
Contact your health care provider right away if you get any symptoms that concern you, even several months after getting the vaccine.
For a more complete list of side effects, ask your health care provider.
What are the ingredients in GARDASIL?
The ingredients are proteins of HPV Types 6, 11, 16, and 18, amorphous aluminum hydroxyphosphate sulfate, yeast protein, sodium chloride, L-histidine, polysorbate 80, sodium borate, and water for injection.
This leaflet is a summary of information about GARDASIL. If you would like more information, please talk to your health care provider or visit www.gardasil.com.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 0.5 mL Vial Carton
NDC 0006-4045-00
1 Single-dose Vial 0.5 mLREFRIGERATE
[Human Papillomavirus
Quadrivalent
(Types 6, 11, 16, and 18)
Vaccine, Recombinant]
GARDASIL®0.5 mL dose contains human
papillomavirus L1 protein of each
type adsorbed on amorphous
aluminum hydroxyphosphate
sulfate adjuvant as follows:
Type 6 (20 mcg), Type 11 (40 mcg),
Type 16 (40 mcg), Type 18 (20 mcg)Vaccine is prepared from
fermentation cultures of a
recombinant strain of yeast
Saccharomyces cerevisiae
containing the genes for the
human papillomavirus L1 protein
of each type (6, 11, 16, 18).Contains no preservative
or antibiotics.Rx only
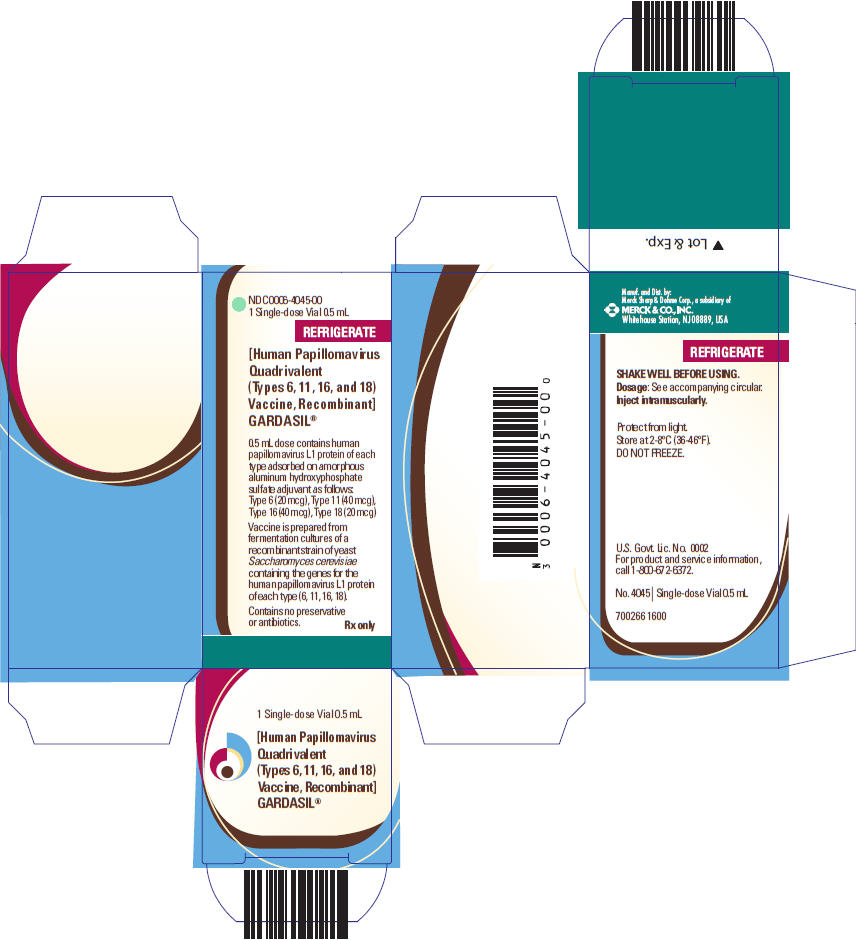
-
PRINCIPAL DISPLAY PANEL - 10 Single-Dose 0.5 mL Syringe Carton
NDC 0006-4109-02
10 Single-dose 0.5-mL SyringesREFRIGERATE
[Human Papillomavirus Quadrivalent
(Types 6, 11, 16, and 18)
Vaccine, Recombinant]
GARDASIL®Each 0.5-mL dose contains human papillomavirus L1 protein of
each type adsorbed on amorphous aluminum hydroxyphosphate
sulfate adjuvant as follows:
Type 6 (20 mcg), Type 11 (40 mcg), Type 16 (40 mcg), Type 18 (20 mcg)Vaccine is prepared from fermentation cultures of a recombinant strain
of yeast Saccharomyces cerevisiae containing the genes for the
human papillomavirus L1 protein of each type (6, 11, 16, 18).
Contains no preservative or antibiotics.Rx only
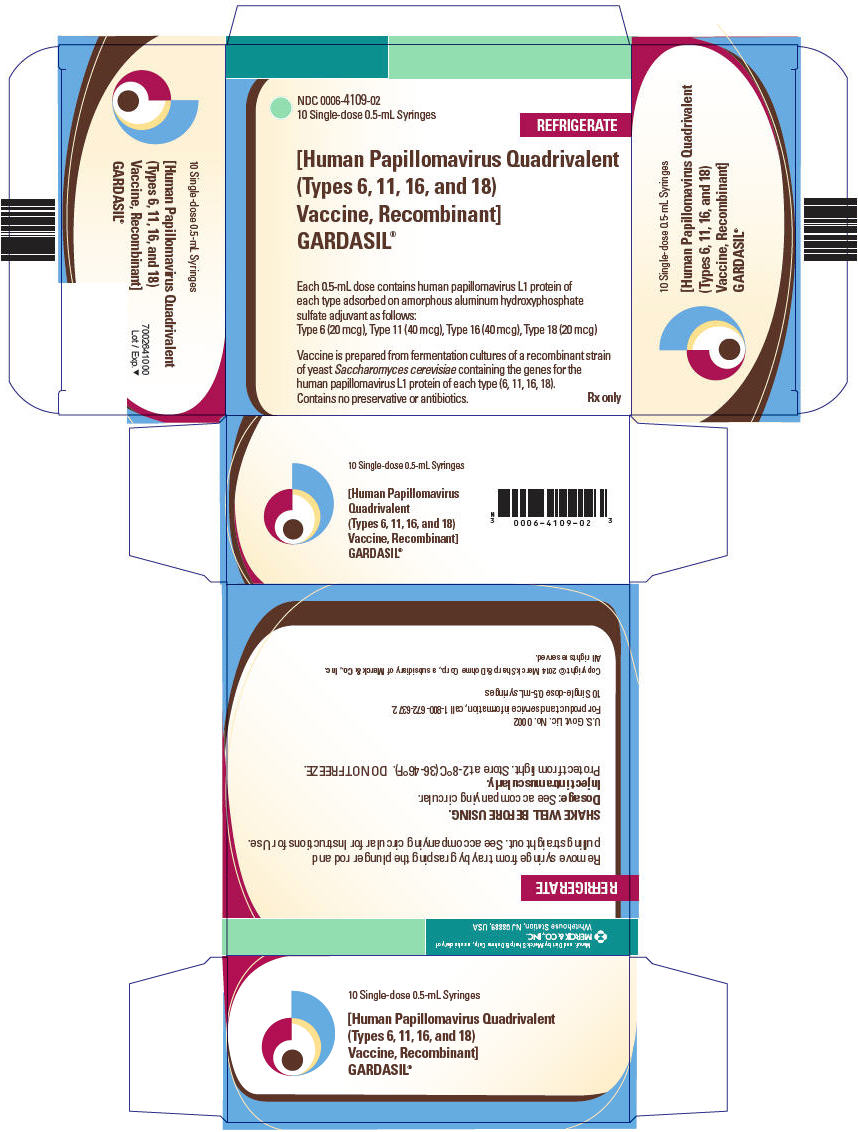
-
INGREDIENTS AND APPEARANCE
GARDASIL
human papillomavirus quadrivalent (types 6, 11, 16, and 18) vaccine, recombinant injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:0006-4045 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN PAPILLOMAVIRUS TYPE 6 L1 CAPSID PROTEIN ANTIGEN (UNII: 61746O90DY) (HUMAN PAPILLOMAVIRUS TYPE 6 L1 CAPSID PROTEIN ANTIGEN - UNII:61746O90DY) HUMAN PAPILLOMAVIRUS TYPE 6 L1 CAPSID PROTEIN ANTIGEN 20 ug in 0.5 mL HUMAN PAPILLOMAVIRUS TYPE 11 L1 CAPSID PROTEIN ANTIGEN (UNII: Z845VHQ61P) (HUMAN PAPILLOMAVIRUS TYPE 11 L1 CAPSID PROTEIN ANTIGEN - UNII:Z845VHQ61P) HUMAN PAPILLOMAVIRUS TYPE 11 L1 CAPSID PROTEIN ANTIGEN 40 ug in 0.5 mL HUMAN PAPILLOMAVIRUS TYPE 16 L1 CAPSID PROTEIN ANTIGEN (UNII: 6LTE2DNX63) (HUMAN PAPILLOMAVIRUS TYPE 16 L1 CAPSID PROTEIN ANTIGEN - UNII:6LTE2DNX63) HUMAN PAPILLOMAVIRUS TYPE 16 L1 CAPSID PROTEIN ANTIGEN 40 ug in 0.5 mL HUMAN PAPILLOMAVIRUS TYPE 18 L1 CAPSID PROTEIN ANTIGEN (UNII: J2D279PEM5) (HUMAN PAPILLOMAVIRUS TYPE 18 L1 CAPSID PROTEIN ANTIGEN - UNII:J2D279PEM5) HUMAN PAPILLOMAVIRUS TYPE 18 L1 CAPSID PROTEIN ANTIGEN 20 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 0.78 mg in 0.5 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 50 ug in 0.5 mL SODIUM BORATE (UNII: 91MBZ8H3QO) 35 ug in 0.5 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 9.56 mg in 0.5 mL WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity ADJV ALUMINUM HYDROXYPHOSPHATE SULFATE (UNII: F41V936QZM) 225 ug in 0.5 mL Product Characteristics Color WHITE (white, cloudy) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-4045-00 1 in 1 CARTON 1 NDC:0006-4045-01 0.5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 2 NDC:0006-4045-41 10 in 1 CARTON 2 NDC:0006-4045-01 0.5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125126 06/08/2006 GARDASIL
human papillomavirus quadrivalent (types 6, 11, 16, and 18) vaccine, recombinant injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:0006-4109 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN PAPILLOMAVIRUS TYPE 6 L1 CAPSID PROTEIN ANTIGEN (UNII: 61746O90DY) (HUMAN PAPILLOMAVIRUS TYPE 6 L1 CAPSID PROTEIN ANTIGEN - UNII:61746O90DY) HUMAN PAPILLOMAVIRUS TYPE 6 L1 CAPSID PROTEIN ANTIGEN 20 ug in 0.5 mL HUMAN PAPILLOMAVIRUS TYPE 11 L1 CAPSID PROTEIN ANTIGEN (UNII: Z845VHQ61P) (HUMAN PAPILLOMAVIRUS TYPE 11 L1 CAPSID PROTEIN ANTIGEN - UNII:Z845VHQ61P) HUMAN PAPILLOMAVIRUS TYPE 11 L1 CAPSID PROTEIN ANTIGEN 40 ug in 0.5 mL HUMAN PAPILLOMAVIRUS TYPE 16 L1 CAPSID PROTEIN ANTIGEN (UNII: 6LTE2DNX63) (HUMAN PAPILLOMAVIRUS TYPE 16 L1 CAPSID PROTEIN ANTIGEN - UNII:6LTE2DNX63) HUMAN PAPILLOMAVIRUS TYPE 16 L1 CAPSID PROTEIN ANTIGEN 40 ug in 0.5 mL HUMAN PAPILLOMAVIRUS TYPE 18 L1 CAPSID PROTEIN ANTIGEN (UNII: J2D279PEM5) (HUMAN PAPILLOMAVIRUS TYPE 18 L1 CAPSID PROTEIN ANTIGEN - UNII:J2D279PEM5) HUMAN PAPILLOMAVIRUS TYPE 18 L1 CAPSID PROTEIN ANTIGEN 20 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 0.78 mg in 0.5 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 50 ug in 0.5 mL SODIUM BORATE (UNII: 91MBZ8H3QO) 35 ug in 0.5 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 9.56 mg in 0.5 mL WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity ADJV ALUMINUM HYDROXYPHOSPHATE SULFATE (UNII: F41V936QZM) 225 ug in 0.5 mL Product Characteristics Color WHITE (white, cloudy) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-4109-09 6 in 1 CARTON 1 NDC:0006-4109-01 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:0006-4109-02 10 in 1 CARTON 2 NDC:0006-4109-01 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125126 06/08/2006 Labeler - Merck Sharp & Dohme LLC (118446553)