Label: ZYLET- loteprednol etabonate and tobramycin suspension/ drops
- NDC Code(s): 24208-358-01, 24208-358-05, 24208-358-10
- Packager: Bausch & Lomb Incorporated
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 30, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZYLET safely and effectively. See full prescribing information for ZYLET. ZYLET® (loteprednol etabonate and tobramycin ophthalmic ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE ZYLET® (loteprednol etabonate and tobramycin ophthalmic suspension), 0.5%/0.3% is a topical anti-infective and corticosteroid combination for steroid-responsive inflammatory ocular conditions for ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Recommended Dosing - Apply one or two drops of ZYLET into the conjunctival sac of the affected eye every four to six hours. During the initial 24 to 48 hours, the dosing may be increased, to ...
-
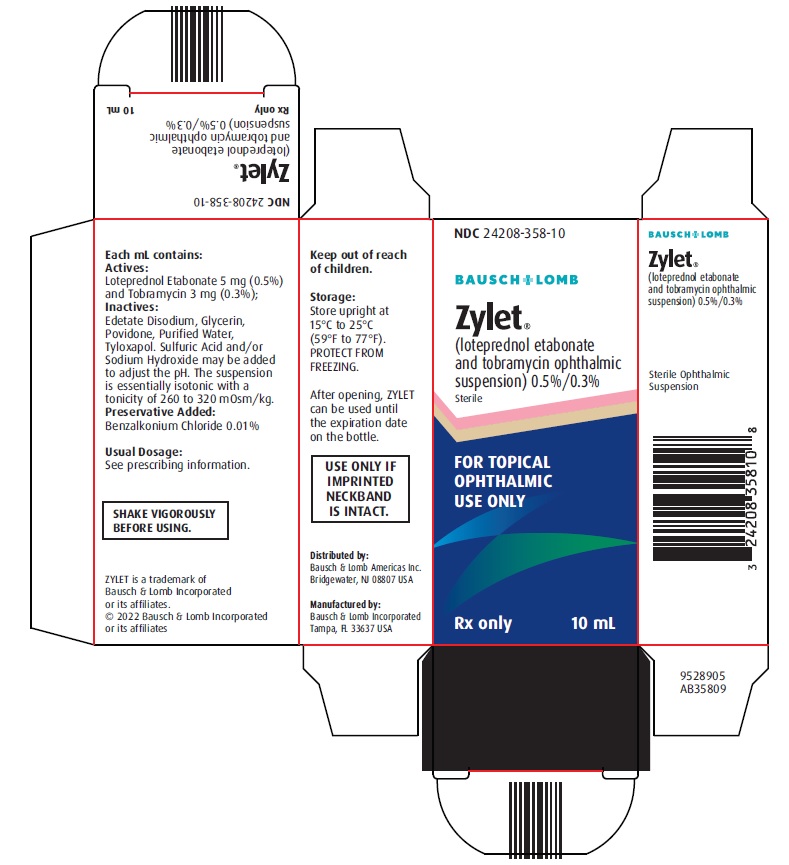
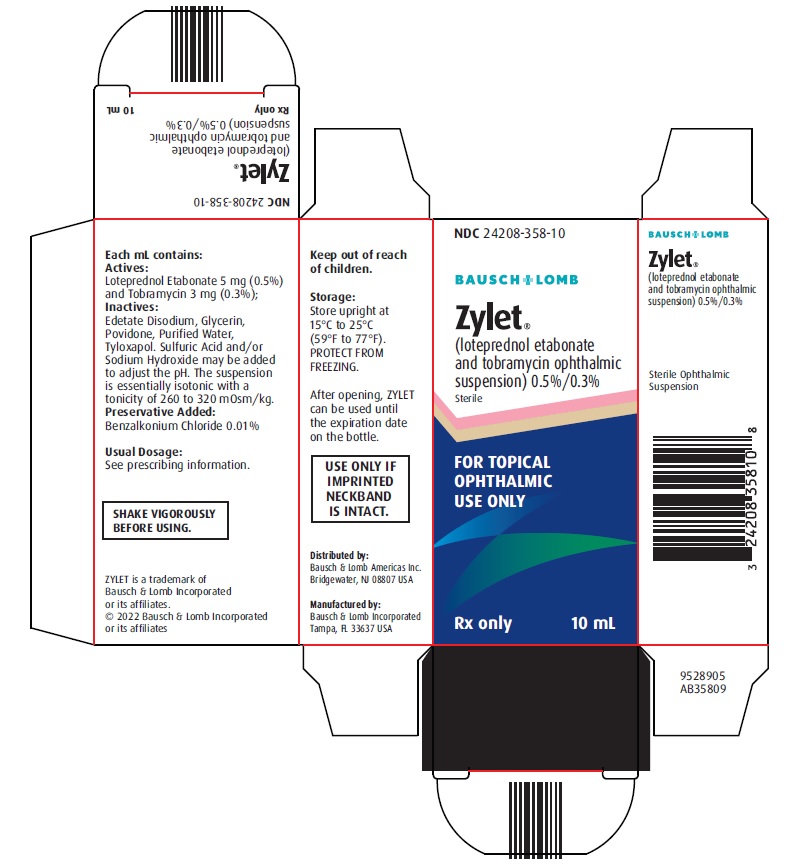
3 DOSAGE FORMS AND STRENGTHS Ophthalmic suspension containing 5 mg/mL (0.5%) loteprednol etabonate and 3 mg/mL (0.3%) tobramycin.
-
4 CONTRAINDICATIONS 4.1 Nonbacterial Etiology - ZYLET, as with other steroid anti-infective ophthalmic combination drugs, is contraindicated in most viral diseases of the cornea and conjunctiva including epithelial ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Intraocular Pressure (IOP) Increase - Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision. Steroids should ...
-
6 ADVERSE REACTIONS Adverse reactions have occurred with steroid/anti-infective combination drugs which can be attributed to the steroid component, the anti-infective component, or the combination. ZYLET - In a ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies with loteprednol etabonate or tobramycin in pregnant women. Loteprednol etabonate produced teratogenicity at ...
-
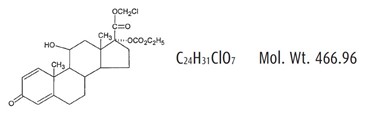
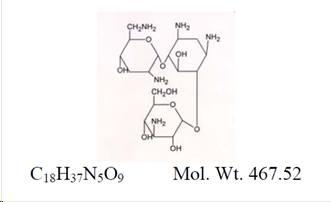
11 DESCRIPTION ZYLET (loteprednol etabonate and tobramycin ophthalmic suspension) is a sterile, multiple dose topical anti-inflammatory corticosteroid and anti-infective combination for ophthalmic use. Both ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Corticosteroids inhibit the inflammatory response to a variety of inciting agents and probably delay or slow healing. They inhibit the edema, fibrin deposition ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term animal studies have not been conducted to evaluate the carcinogenic potential of loteprednol etabonate or ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING ZYLET (loteprednol etabonate and tobramycin ophthalmic suspension) 0.5%/0.3% is supplied in a white low density polyethylene plastic bottle with a white controlled drop tip and a white ...
-
17 PATIENT COUNSELING INFORMATION Risk of Contamination - This product is sterile when packaged. Advise patients not to allow the dropper tip to touch any surface, as this may contaminate the suspension. Risk of Secondary ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 24208-358-10 - BAUSCH + LOMB - Zylet® (loteprednol etabonate - and tobramycin ophthalmic - suspension) 0.5%/0.3% Sterile - FOR TOPICAL - OPHTHALMIC - USE ONLY - Rx only - 10 ...
-
INGREDIENTS AND APPEARANCEProduct Information