Label: ZYDELIG- idelalisib tablet, film coated
- NDC Code(s): 61958-1701-1, 61958-1702-1
- Packager: Gilead Sciences, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 20, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZYDELIG safely and effectively. See full prescribing information for ZYDELIG. ZYDELIG® (idelalisib) tablets, for oral use ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: FATAL AND SERIOUS TOXICITIES: HEPATIC, SEVERE DIARRHEA, COLITIS, PNEUMONITIS, INFECTIONS, and INTESTINAL PERFORATION
Fatal and/or serious hepatotoxicity occurred in 16% of Zydelig-treated patients. Monitor hepatic function prior to and during treatment. Interrupt and then reduce or discontinue Zydelig as recommended [see Dosage and Administration (2.2), Warnings and Precautions (5.1)].
Fatal and/or serious and severe diarrhea or colitis occurred in 20% of Zydelig-treated patients. Monitor for the development of severe diarrhea or colitis. Interrupt and then reduce or discontinue Zydelig as recommended [see Dosage and Administration (2.2), Warnings and Precautions (5.2)].
Fatal and/or serious pneumonitis occurred in 4% of Zydelig-treated patients. Monitor for pulmonary symptoms and bilateral interstitial infiltrates. Interrupt or discontinue Zydelig as recommended [see Dosage and Administration (2.2), Warnings and Precautions (5.3)].
Fatal and/or serious infections occurred in 48% of Zydelig-treated patients. Monitor for signs and symptoms of infection. Interrupt Zydelig if infection is suspected [see Dosage and Administration (2.2), Warnings and Precautions (5.4)].
Fatal and serious intestinal perforation can occur in Zydelig-treated patients across clinical trials. Discontinue Zydelig for intestinal perforation [see Warnings and Precautions (5.5)].
Close -
1 INDICATIONS AND USAGEZydelig is indicated, in combination with rituximab, for the treatment of patients with relapsed chronic lymphocytic leukemia (CLL) for whom rituximab alone would be considered appropriate therapy ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage of Zydelig is 150 mg administered orally twice daily with or without food until disease progression or unacceptable toxicity. The optimal and safe ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 100 mg: orange, oval-shaped, film-coated tablet debossed with "GSI" on one side and "100" on the other side. 150 mg: pink, oval-shaped, film-coated tablet debossed with "GSI" on one side ...
-
4 CONTRAINDICATIONSZydelig is contraindicated in patients with a history of serious hypersensitivity reactions to idelalisib, including anaphylaxis, or patients with a history of toxic epidermal necrolysis with any ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hepatotoxicity - Fatal and/or serious hepatotoxicity occurred in 16% of patients treated with Zydelig in combination with rituximab or with unapproved combination therapies. Elevations in ALT ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling. Hepatotoxicity [see Warnings and Precautions (5.1)] Severe Diarrhea or Colitis [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Effects of Other Drugs on Zydelig - Table 6 lists the potential effects of the coadministration of strong CYP3A modulators on Zydelig. Table 6 Drug Interactions with Zydelig that affect ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings in animal studies and the mechanism of action [see Clinical Pharmacology (12.1)], Zydelig may cause fetal harm when administered to a pregnant ...
-
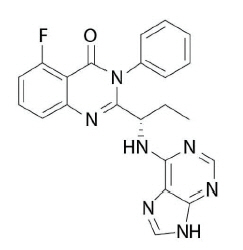
11 DESCRIPTIONIdelalisib is a kinase inhibitor. The chemical name for idelalisib is 5-fluoro-3-phenyl-2-[(1S)-1-(9H-purin-6-ylamino)propyl]quinazolin-4(3H)-one. It has a molecular formula of C22H18FN7O and a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Idelalisib is an inhibitor of phosphatidylinositol 3-kinase, PI3Kδ, which is expressed in normal and malignant B-cells. Idelalisib induced apoptosis and inhibited ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Idelalisib was not carcinogenic in a 26-week study in transgenic mice when administered daily by oral gavage at doses up to 500 ...
-
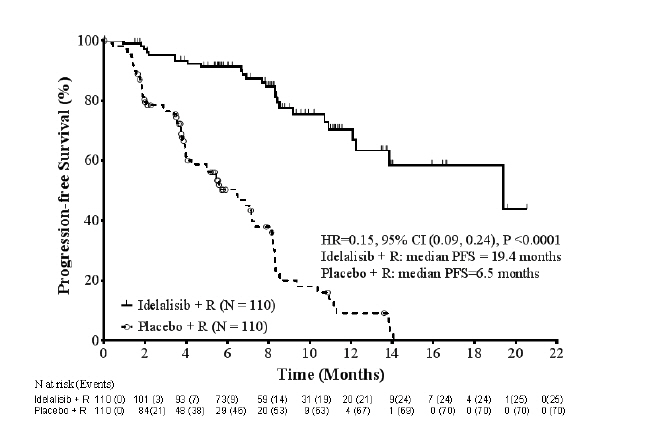
14 CLINICAL STUDIESZydelig was evaluated in a randomized, double-blind, placebo-controlled study GS-US-312-0116 (referred to as 312-0116) (NCT01539512) in 220 patients with relapsed CLL who required treatment and ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGZydelig tablets supplied as follows: Tablet StrengthPackage ConfigurationNDC No.Description of Tablet; Debossed on Tablet - 150 mgHigh density polyethylene (HDPE) bottle with a polyester ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Hepatotoxicity - Advise patients that Zydelig can cause significant elevations in liver enzymes, and that serial ...
-
SPL UNCLASSIFIED SECTIONManufactured and distributed by: Gilead Sciences, Inc. Foster City, CA 94404 - GSI and Zydelig are trademarks or registered trademarks of Gilead Sciences, Inc., or its related companies. All other ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration.Revised: 2/2022 - MEDICATION GUIDE - ZYDELIG® (zye-DEL-ig) (idelalisib) tablets - What is the most important ...
-
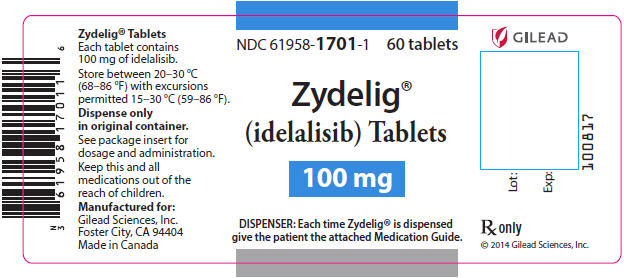
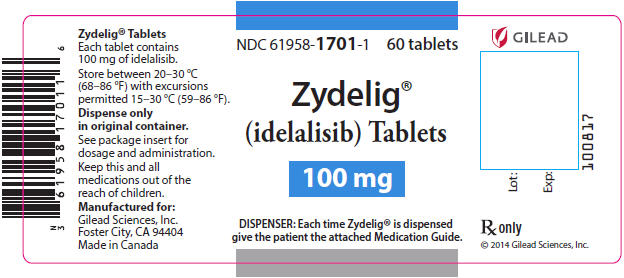
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle LabelNDC 61958-1701-1 - 60 tablets - Zydelig® (idelalisib) Tablets - 100 mg - DISPENSER: Each time Zydelig® is dispensed - give the patient the attached Medication Guide.
-
PRINCIPAL DISPLAY PANEL - 150 mg Tablet Bottle LabelNDC 61958-1702-1 - 60 tablets - Zydelig® (idelalisib) Tablets - 150 mg - DISPENSER: Each time Zydelig® is dispensed - give the patient the attached Medication Guide.
-
INGREDIENTS AND APPEARANCEProduct Information